Toward a Biosignature for Suicide
Abstract
Objective:
Method:
Results:
Conclusions:
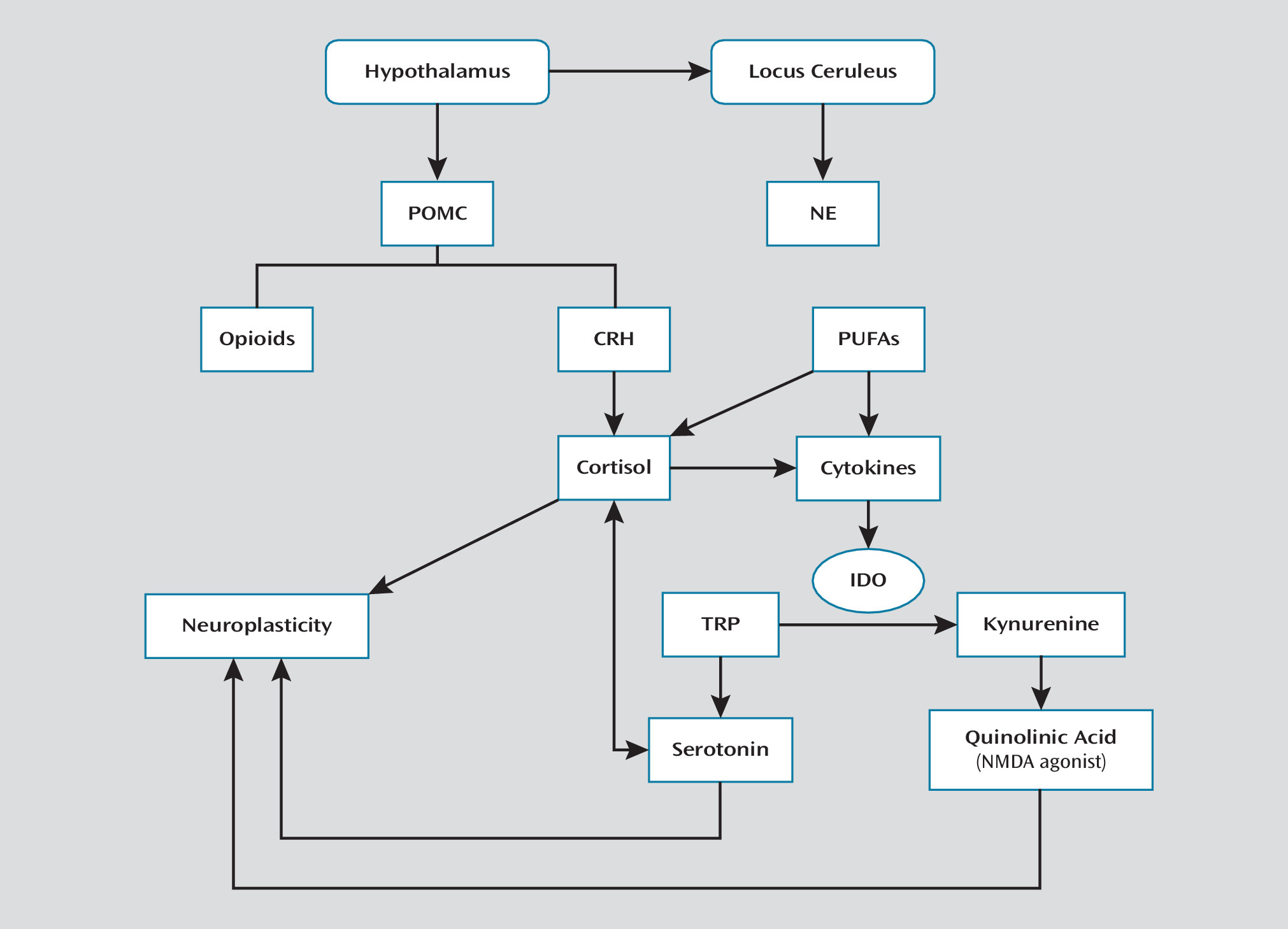
Stress Response and Its Modulation
Hypothalamic-Pituitary-Adrenal Axis and Locus Ceruleus-Norepinephrine System
| System | Paper | mRNA | Brain Regions | Findings |
|---|---|---|---|---|
| CRH-HPA | Pandey et al. (15) | GR-α, GR-β, MR | Amygdala, PFC, hippocampus | In teenage suicide victims compared with deceased healthy controls: 1) GR-α mRNA levels reduced in amygdala and PFC, but not hippocampus, 2) no difference in PFC GR-β mRNA expression, 3) no difference in MR mRNA expression in all three brain regions |
| López et al. (16) | GR, MR | Hippocampus | No difference in GR or MR mRNA levels in depressed suicide victims compared with deceased controls | |
| Labonte et al. (19) | GR, GR exon splice variants (1B, 1C, 1H) | Hippocampus, ACC | Hippocampus: decreased GR, GR1B, GR1C, and GR1H mRNA expression in suicide victims with abuse history compared with suicide victims without abuse history and controls; no difference between suicide victims without abuse and controls in GR1B, GR1C, and GR1H levels; ACC: no difference between groups in GR and variant expression | |
| López et al. (14) | POMC, GR | Pituitary | Increased POMC mRNA density in corticotropic cells of suicide victims compared with controls; no difference in GR mRNA expression | |
| Sequeira et al. (112) | MT | ACC, nucleus accumbens | Decreased mRNA expression of two MT families, MT-1 and MT-2, in both ACC and nucleus accumbens of depressed suicide victims compared with deceased controls; no difference in MT-3 mRNA expression | |
| Merali et al. (13) | CRH1, CRH2 | FPC, DMPFC, VLPFC | In FPC, CRH1 mRNA expression lowered in suicide victims compared with controls, no difference in CRH2 mRNA levels; no differences in either CRH1 or CRH2 mRNA expression in DMPFC or VLPFC | |
| NE-LC | Du et al. (51) | COMT | FPC, OFC | COMT mRNA expression elevated in depressed suicide victims compared with deceased controls in both the FPC and OFC; also, Met carriers who died of suicide showed elevated COMT mRNA levels in FPC compared with control Met carriers |
| Escribá et al. (76) | α2A-adrenoceptor | PFC | Increased mRNA expression levels found in suicide victims compared with controls | |
| Cytokines | Tonelli et al. (61) | TNF-α, IL-1β, IL-4, IL-5, IL-6, IL-13 | BA 11 | IL-4 mRNA expression was increased in female suicide victims compared with controls, whereas IL-13 mRNA expression was elevated in male suicide victims; no difference in the remaining factors |
| Endogenous opioids | Escribá et al. (76) | μ-opioid receptor | PFC | Increased mRNA expression found in suicide victims compared with controls |
| Hurd et al. (77) | Prodynorphin | Caudate nucleus | Increased mRNA expression levels in suicide victims compared with patients with schizophrenia and controls | |
| Serotonin | Bach-Mizrachi et al. (85) | TPH2 | DRN, MRN | Higher mRNA expression in DRN (along entire rostrocaudal axis) and MRN of suicide victims compared with deceased nonpsychiatric controls |
| Bach-Mizrachi et al. (88) | TPH2 | DRN, MRN | Mean TPH2 mRNA expression levels per neuron in DRN and MRN greater in depressed suicide victims than deceased healthy controls; in DRN, expression levels different in caudal but not rostral regions | |
| De Luca et al. (87) | TPH2 | DLPFC (BA 46) | No difference in mRNA expression between suicide victims and controls | |
| Perroud et al. (84) | TPH1, TPH2 | VPFC | Increased TPH2 mRNA expression in suicide victims compared with controls; no difference in TPH1 expression | |
| López et al. (16) | 5-HTR1A | Hippocampus | Decreased mRNA expression in depressed suicide victims compared with deceased controls | |
| Matthews and Harrison (89) | 5-HTR1A | DRN | No difference in mRNA expression between suicide victims, three non-suicide groups (schizophrenia, bipolar disorder, depression) and controls | |
| Escribá et al. (76) | 5-HTR1A, 5-HTR2A | PFC | Increased mRNA expression of both 5-HTR1A and 5-HTR2A in suicide victims compared with controls | |
| Sequeira et al. (112) | 5-HTR2A, PER1, ADCY1 | DLPFC | Decreased mRNA expression of 5-HTR2A, ADCY1 (adenylate cyclase gene) and PER1 (period homologue) in suicide victims compared with deceased controls | |
| Pandey et al. (113) | 5-HTR2C | Multiple brain regions | No difference in mRNA expression levels of 5-HTR2C between suicide victims and controls in any studied brain regions (PFC, hippocampus, choroid plexus, hypothalamus, nucelus accumbens, cerebellum) | |
| GABA | Merali et al. (13) | GABAA | FPC, DMPFC, VLPFC | In FPC, mRNA levels of GABAA α1, α3, α4, and δ subunits, but not α2, α5, or λ, were lower in suicide victims compared with deceased controls; no difference in GABAA expression between groups in DMPFC or VLPFC |
| Poulter et al. (140) | GABAA | OFC, LC, PVN, hippocampus, amygdala | Pattern of GABAA subunit expression differed in suicide victims compared with controls; lowered subunit coordination in hippocampus and amygdala, increased coordination in OFC and PVN, unchanged in LC | |
| Sequeira et al. (139) | Multiple GABA-ergic genes | 17 brain regions | Up-regulation in mulitple GABA-ergic genes in depressed suicide victims, but no differences between nondepressed suicide victims and controls observed | |
| Klempan et al. (143) | Multiple GABA-ergic genes | VPFC (BA 44, 45, 46, 47), DPFC | Multiple GABA-ergic genes (GABAA α5, β1, δ, γ1, and γ2, GABBR2, SLC6A1) differentially expressed in BA 46 of suicide victims compared with controls | |
| Choudary et al. (150) | Multiple GABA-ergic genes | ACC | GABAA α1 and GABAA β3 mRNA expression up-regulated in suicide victims with mood disorders compared with controls | |
| Glutamate | Sequeira et al. (139) | Multiple glutamatergic genes | Multiple brain regions | GLUL down-regulated in PFC, amygdala of depressed suicide victims; AMPA up-regulated in amygdala, hippocampus, nucleus accumbens, and few cortical regions in depressed suicide victims; GRM3 down-regulated in PFC, parietal cortex of suicide victims (with and without depression) compared with controls |
| Klempan et al. (143) | Multiple glutamatergic genes | VPFC (BA 44, 45, 46, 47), DPFC | Multiple glutamatergic genes (GRIA3, GRIN2A, SLC1A2) showed increased expression in BA 46 of suicide victims compared with controls | |
| Neuronal plasticity | Pandey et al. (162) | BDNF, TrkB | PFC (BA 9), hippocampus | BDNF and TrkB mRNA levels lower in PFC and hippocampus of teenage suicide victims compared with deceased healthy controls |
| Dwivedi et al. (164) | BDNF, TrkB | PFC (BA 9), hippocampus | BDNF and TrkB mRNA expression lower in PFC and hippocampus of suicide victims compared with deceased healthy controls | |
| Keller et al. (170) | BDNF promoter/exon IV | Wernicke area | Suicide victims with high BDNF promoter/exon IV methylation patterns showed decreased BDNF mRNA expression compared with intermediate and low methylation pattern groups (including suicide victims and controls) | |
| Keller et al. (169) | TrkB, TrkB-T1 | Wernicke area | No differences in mRNA expression levels of either TrkB or TrkB-T1 in suicide victims compared with deceased controls | |
| Dwivedi et al. (166) | TrkA, TrkC, p75(NTR) | PFC, hippocampus | TrkA mRNA expression decreased, whereas p75(NTR) expression increased, in both PFC and hippocampus of suicide victims compared with deceased nonpsychiatric controls; TrkC expression decreased in hippocampus | |
| Dwivedi et al. (167) | NGF, NT-3, NT-4/5, NSE | PFC, hippocampus | NGF, NT-3, NT-4/5, and NSE mRNA expression levels lower in hippocampus of suicide victims compared with deceased controls; in PFC, only NT-4/5 mRNA expression lowered in suicide victims | |
| Methylation | Labonte et al. (19) | GR, GR exon splice variants (1B, 1C, 1H) | Hippocampus | GR1B: hypermethylation at CpG6 and CpG8, hypomethylation at CpG11 in suicide victims compared with controls; GR1C: hypermethylation at CpG9 and CpG12 (suicide victims), CpG8 (abused suicide victims), hypomethylation at CpG13 (abused suicide victims); GR1H: hypomethylation in abused suicide victims (CpG2, CpG5, CpG10) compared with suicide victims without abuse (CpG3, CpG7, CpG8, CpG12) and controls (CpG1) |
| Poulter et al. (142) | GABAA α1, DNMT | Multiple brain regions | DNMT-3B up-regulation associated with hypermethylation at three sites on GABAA α1 gene in FPC of depressed suicide victims compared with deceased controls | |
| Keller et al. (169) | TrkB, TrkB-T1, BDNF | Wernicke area | No differences in methylation patterns at any TrkB or TrkB-T1 CpG sites examined in suicide victims compared with deceased controls; hypermethylation of BDNF promoter IV present in suicide | |
| Keller et al. (170) | BDNF promoter/exon IV | Wernicke area | Significant hypermethylation at two CpG sites of BDNF promoter/exon IV in suicide victims compared with deceased controls |
HPA axis in suicide.
| Systema | Genes | Paper | Sample | Findings |
|---|---|---|---|---|
| CRH-HPA | FKBP5 | Supriyanto et al. (25) | Japanese | Higher frequency of TC haplotype associated with higher transcription of FKBP5 in suicide victims compared with live nonpsychiatric controls |
| NE-LC | ADRA2 | Fukutake et al. (37) | Japanese | C-allele of C-1291G SNP (unknown function) more prevalent in female suicide victims than in live female nonpsychiatric controls |
| MAO-A | Du et al. (46) | Hungarian | High activity-related allele of EcoRV polymorphism differed in depressed male but not female suicide victims compared with deceased nonpsychiatric controls | |
| COMT | Ono et al. (48) | Japanese | High activity Val/Val genotype of 158Val/Met polymorphism found less prevalent and Val/Met genotype more prevalent in male but not female suicide victims compared with living nonpsychiatric controls | |
| COMT | Du et al. (51) | Hungarian | No difference in allele frequency or genotypic distribution in depressed suicide victims compared with deceased nonpsychiatric controls | |
| COMT | Pivac et al. (49) | Slovenian | Val/Val and Val/Met genotypes of 158Val/Met polymorphism more prevalent in male but not female suicide victims, as compared with deceased controls | |
| Endogenous opioid | OPRM1 | Hishimoto et al. (71) | Japanese | The A/A genotype of the A118G SNP, linked to increased binding was more frequent in suicide victims than in live healthy controls |
| Serotonin | TPH2 | Zill et al. (91) | German | SNP rs1386494 (located between exon 5 and exon 7) associated with suicide in a sample of suicide victims compared with live healthy controls |
| TPH2 | Lopez de Lara et al. (92) | French-Canadian | T allele of SNP rs4448731 and G allele of rs6582071 in upstream region, G allele of rs4641527 in intron 1, and C allele of rs1386497 in intron 8 more prevalent in depressed suicide victims compared with live depressed patients | |
| TPH2 | Stefulj et al. (93) | Croatian | No difference in genotype or allele frequency of G-703T SNP in sample of suicide victims compared with controls | |
| TPH2 | Mouri et al. (94) | Japanese | No difference in genotype or allele frequency of 15 tagging SNPs in suicide victims compared with live healthy controls | |
| TPH1, TPH2, SLC6A4 | Buttenschøn et al. (102) | Danish | No significant association of either TPH1, TPH2, or 5-HTTLPR with suicide in a sample of suicide victims compared with living controls | |
| TPH, 5-HTT | Jernej et al. (103) | Croatian | Co-occurrence of 5-HTT intron 2 VNTR 10 allele and CC variant of TPH intron 7 A218C SNP more frequent in suicide victims than in live healthy controls | |
| 5-HTT | Bondy et al. (171) | German | S allele and homozygous SS genotype of 5-HTTLPR more prevalent in suicide victims than in healthy living controls | |
| 5-HTT | Lopez de Lara et al. (101) | French-Canadian | VNTR STin2 allele 10 more frequent in depressed suicide victims compared with depressed controls; no significant genotypic or allelic differences noted at 5-HTTLPR locus | |
| 5-HTT | Hranilovic et al. (105) | Croatian | No difference in allele and genotype frequency in either 5-HTTLPR or VNTR intron 2 polymorphisms in suicide victims compared with live healthy controls | |
| 5-HTR1A | Videtic et al. (172) | Slovenian | No difference in C-1019G allele or genotype distributions between suicide victims and deceased controls | |
| 5-HTR1B | Zouk et al. (114) | French-Canadian | Higher frequency of T allele at A-161T locus in suicide victims compared with live nonpsychiatric controls; no differences at T-261G, C129T, G861C, or A1180G loci | |
| 5-HTR1B, 5-HTR1Dα, 5-HTR1E, 5-HTR1F, 5-HTR2C, 5-HTR5A, 5-HTR6 | Turecki et al. (117) | French-Canadian | No difference in allele or genotype frequencies in any loci examined (G861C of 5-HTR1B, T1350C of 5-HTR1Dα, C117T of 5-HTR1E, C-78T and C528T of 5-HTR1F, G-995A of 5-HTR2C, G-19C of 5-HTR5A, and C267T of 5-HTR6) in suicide victims compared with live healthy controls | |
| 5-HTR2A | Ono et al. (119) | Japanese | No association between 5-HTR2A A-1438G SNP and suicide | |
| 5-HTR2A | Turecki et al. (111) | French-Canadian | No difference in T102C or A-1438G SNP allele or genotype distribution in suicide victims compared with deceased controls | |
| 5-HTR2C | Videtic et al. (116) | Slovenian | Increased frequency of G68C G-allele and GG genotype in suicide victims compared with controls; relationship also significant in females but not males; no association between G-995A and suicide | |
| 5-HTR6 | Azenha et al. (115) | Portuguese | Difference in C267T genotype distribution in male suicide victims compared with deceased nonpsychiatric controls; no association present when females included | |
| Neuronal plasticity | BDNF | Pregelj et al. (168) | Slovenian | No difference in Val66Met variant distribution in suicide victims compared with deceased controls; in females, more likely to have Met alleles associated with low activity |
| DISC1 | Ratta-apha et al. (173) | Japanese | Allele frequency and genotype distribution of Ser704Cys variant differ in suicide victims and live healthy controls |
Noradrenergic-locus ceruleus system in suicide.
Neuroinflammation
Cytokines.
Cytokine abnormalities in suicide.
Polyunsaturated fatty acids.
Polyunsaturated fatty acid status in suicide.
Endogenous Opioid System
The opioid system in suicide.
Neurotransmitter Systems Implicated in Suicide
| Systema | Focus of Findings | Findings in Suicide | Locationb | Samplesc | |
|---|---|---|---|---|---|
| Neurochemical findings | |||||
| System | Compound | Findings in suicide | Location | Samples | |
| CRH-HPA | CRH | Elevated concentration | CSF (9), LC (10, 11), caudal raphe nucleus (10), DLPFC, VMPFC (11), FPPFC (11, 13), DMPFC (13) | DS v. NC | |
| Reduced concentration | DVC (11) | DS v. NC | |||
| Vasopressin | Elevated concentration | LC, DMPFC, paraventricular hypothalamic nucelus (11) | DS v. NC | ||
| Reduced concentration | DVC (11) | DS v. NC | |||
| CRHR1 | Reduction in number of binding sites | Frontal cortex (12) | DS v. NC | ||
| Cortisol | Reduced baseline levels | Plasma (20) | MDS v. PC | ||
| NE-LC | Tyrosine hydroxylase | Reduced stain density | LC (31) | ||
| NE | Elevated concentration | Temporal cortex (33) | |||
| MAO-A, MAO-B | No difference | Frontal cortex (47) | |||
| MHPG | Reduced concentration | CSF (20) | MDS v. PC | ||
| Cytokines | IL-1β, IL-6, TNFα | Elevated expression | BA 10 (60) | ||
| Microglia | Elevated density | DLPFC, ACC, MDT (62) | |||
| Fatty acids | DHA | Reduced concentration | Serum (66) | ||
| DHA, MUFA, saturated FA | No difference | BA 10 (67) | |||
| FAs | No difference | OFC, VPFC (68) | S v. DS v. PC | ||
| Endogenous opioids | β-endorphin | Reduced concentration | Temporal cortex, frontal cortex, caudate nucleus (78) | ||
| Serotonin | TPH | Elevated concentration | DRN (86) | ||
| No difference | MRN (86) | ||||
| 5-HT | Elevated concentration | Brainstem (90) | |||
| 5-HIAA | Reduced concentration | CSF (121–124) | S v. SA (121); MDS v. PC (122, 124) | ||
| Elevated concentration | Hippocampus (125), amygdala (126) | DS v. NC (126) | |||
| Dopamine | HVA/5-HIAA | Higher ratio | Frontal cortex (134) | ||
| DOPAC | Reduced concentration | Caudate, putamen (AFS), nucelus accumbens (AFS) (135) | AFS v. ATS v. NC | ||
| Glutamate/GABA | Glutamine | Reduced concentration | Hypothalamus (146) | ||
| Neuronal plasticity | BDNF | Reduced concentration | PFC, hippocampus (165) | ||
| NT-3 | Reduced concentration | Hippocampus (165) | |||
| Autoradiographic findings | |||||
| System | Receptor | Ligand | Findings in suicide | Location | Samples |
| NE-LC | α1-adrenoceptor | [3H]Prazosin | Decreased binding | Caudate nucleus, PFC, frontoparietal and temporal cortical regions (32) | |
| [3H]Prazosin | Increased binding | PFC (33) | |||
| [3H]Prazosin | No difference in number of receptors | PFC, hippocampus, hypothalamus, thalamus, caudate, putamen (36) | ATS v. NC; AFS v. NC | ||
| α2-adrenoceptor | p-[125I]Iodoclonidine | Increased binding | LC (30) | ||
| [3H]UK 14304 | Increased binding | Hippocampus (CA1), frontal cortex (35) | |||
| [3H]Rauwolscine | Decreased binding | Occipital cortex, hippocampus (36) | ATS v. NC | ||
| [3H]Aminoclonidine | No difference | PFC (33) | |||
| [3H]Rauwolscine | Increased binding | Temporal cortex (BA 21/22) (36) | AFS v. NC | ||
| System | Receptor | Ligand | Findings in suicide | Location | Samples |
| β-adrenoceptor | [3H]DHA | Increased binding | Frontal cortex (38), PFC (40) | VS v. NC (38) | |
| [125I]PIN | Increased binding | PFC (39) | |||
| [125I]PIN | Decreased binding | Frontal cortex (41) | |||
| [3H]CGP 12177 | Decreased binding | Temporal cortex (42, 43), thalamus (43) | DS v. NC (42); ATS v. NC (43) | ||
| NET | [3H]Nisoxetine | Decreased binding | LC (45) | DS v. NC | |
| Endogenous opioids | μ-opioid receptor | [3H]DAGO | Higher density | Frontal cortex (72, 75), caudate (72); temporal cortex (75) | Young S v. NC (75) |
| [3H]DAGO | No difference | PFC, PPCG (74) | |||
| DAMGO | No difference | PFC (BA 9) (73) | MDS v. NC | ||
| Serotonin | 5-HTT | [3H]CN-IMI | Decreased binding | VLPFC (95), VPFC (96) | |
| [3H]CN-IMI | No difference | DRN, MRN (99) | DS v. NC | ||
| [3H]Imipramine | Decreased binding | Frontal cortex (97); hippocampus (98); claustrum, insular cortex, PCCG (100) | DS v. NC (98) | ||
| [3H]Imipramine | Increased binding | Hippocampus (100) | |||
| 5-HTR1A | [3H]8-OH-DPAT | Increased binding | VLPFC (95), DRN (106) | DS v. NC (106) | |
| [3H]8-OH-DPAT | Decreased binding | DRN (99, 107), MRN (99) | DS v. NC | ||
| 5-HTR2 | [3H]Spiroperidol | Increased density | Frontal cortex (110) | ||
| [3H]Spiroperidol | Increased binding | Frontal cortex (38) | |||
| [3H]Spiperone | Increased binding | Frontal cortex (109) | |||
| Dopamine | D1 receptors | [3H]SCH23390 | No difference | Caudate nucleus, putamen, and nucleus accumbens (133) | DS v. NC |
| D2 receptors | [3H]Raclopride | No difference | Caudate nucleus (132, 133), putamen, nucleus accumbens (133) | DS v. NC | |
| Dopamine reuptake sites | [3H]WIN 35428 | No difference | Caudate nucleus (131) | DS v. NC | |
| GABA | GABAA receptors | [3H]-Flunitrazepam | No difference | LC (141) | DS v. NC |
| GABAB receptors | [3H]GABA | No difference | Frontal cortex, temporal cortex, hippocampus (144) | DS v. NC | |
| Glutamate | NMDA | [3H]Dizocilpine | No difference | Frontal cortex (148, 151, 152), parietal cortex (148), temporal and occipital cortices, hippocampus, thalamus (152), putamen, caudate nucleus (147, 152), nucleus accumbens (147) | DS v. NC (152); S v. PC, S v. NC (147) |
| [3H]CGP-39653 | Decreased binding | Frontal cortex (151) | |||
| AMPA | [3H]AMPA | Increased binding | Caudate nucleus (149) | ||
| [3H]CNQX | Increased binding | Caudate nucleus (147) | S v. PC, S v. NC | ||
| Morphological findings | |||||
| System | Structure | Findings in suicide | Samples | ||
| CRH-HPA | Adrenal glands | Increased adrenal weight (21–23) | VS v. C (21) | ||
| No difference in adrenal weight (24) | |||||
| NE-LC | Locus ceruleus | Decreased number of neurons, decreased neuron density (50) | |||
| Serotonin | DRN | Increase in DRN area, decrease in 5-HT neuron size, increased 5-HT neuron density (89) | S v. PC | ||
| System | Structure | Findings in suicide | Samples | ||
| Neuronal plasticity | Hippocampus | Decreased number of dentate gyrus granule neurons; increased anterior and mid granule cell layer volume (160) | AFS v. NC | ||
| No difference (161) | SS v. NC | ||||
| Parahippocampus | Decreased right parahippocampal volume (161) | SS v. NC | |||
| PFC | Reduction in cortical and laminar thickness (159) | S v. PC | |||
| Other findings | |||||
| System | Test | Findings in suicide | Samples | ||
| CRH-HPA | Dexamethasone suppression test (DST) | DST nonsuppressors more likely to die by suicide (26–28) | MDP DST suppressors v. nonsuppressors (26, 28) | ||
| MDS DST suppressors v. nonsuppressors (27) | |||||
| Dopamine | Apomorphine challenge | Decreased growth hormone response to apomorphine (129) | DS v. DP | ||
Serotonin
Tryptophan hydroxylase and serotonin synthesis in suicide.
Serotonin transporter in suicide.
Serotonin receptors in suicide.
Serotonin turnover in suicide.
Norepinephrine
Dopamine
Dopamine in suicide.
GABA
GABA in suicide.
Glutamate
Glutamate in suicide.
Molecular Markers of Neuronal Plasticity in Suicide
Neuronal Plasticity in Suicide
Conclusions
References
Information & Authors
Information
Published In
History
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

