The critical role of norepinephrine in neural systems underlying the generation of major depressive disorder symptoms has been recognized for many years. There have been several studies on the relationship between norepinephrine systems and major depressive disorder. Norepinephrine transporter binding in the locus ceruleus, which reflects the dynamics of norepinephrine (
2), has been found to be decreased in postmortem tissues from patients with major depressive disorder (
3), suggesting the alteration of norepinephrine systems. Postmortem studies of depressed patients have shown altered density and affinity state of α2A-adrenoceptors, which modulate norepinephrine release (
4). Another study found increased β-adrenergic receptor binding in the cortex density of suicide subjects (
5). Other research found that α2- and β1-adrenoceptor densities in the frontal cortex were higher in antidepressant-free patients with major depressive disorder compared with matched comparison subjects (
6). These studies suggest the critical role of norepinephrine in neural systems underlying the generation of major depressive disorder. The norepinephrine transporter has been targeted as a treatment pathway for major depressive disorder (
7). For instance, antidepressants with dual-target sites that include the norepinephrine transporter, such as venlafaxine, milnacipran, and duloxetine, are equivalent or are even more effective than pure selective serotonin reuptake inhibitors in achieving remission (
8).
Method
Participants
Nineteen patients (11 men and eight women; mean age=40.1 years [SD=10.7], range=20–56 years) fulfilling DSM-IV criteria for major depressive disorder were recruited from two affiliated psychiatric hospitals and two clinics in the Chiba, Japan, area. The participants had no other history of major medical illness. S.M., H.T., K.T., and T.N. evaluated lifetime psychiatric disorders based on the Mini-International Neuropsychiatric Interview (
18), and patients were determined to be free of comorbid psychiatric disorders. Clinical characteristics of the patients are summarized in
Table 1. Seventeen patients were categorized with melancholic depression based on the Mini-International Neuropsychiatric Interview, and none of the patients had received psychopharmacologic treatment for at least 1 year before the study. Sixteen of the 19 patients were antidepressant-naive, and the remaining three patients were antidepressant-free at the time of the study; one patient had taken duloxetine 3 years earlier, one had taken sulpiride 2 years earlier, and the other had taken antidepressants (the specific agents were not identified) 5 years earlier. The lifetime number of major depressive episodes ranged from one to two.
Age- and sex-matched healthy subjects (11 men and eight women; mean age=40.7 years [SD=12.2], range=22–61 years) participated in the study as a comparison group. All healthy subjects were free of any somatic, neurological, or psychiatric disorders and had no history of current or previous drug abuse.
Studies were performed and analyzed at the National Institute of Radiological Sciences (Chiba, Japan). All participants provided written informed consent before participating in the study, which was approved by the Radiation Drug Safety Committee and by the institutional review board of the National Institute of Radiological Sciences, and which was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Clinical and Neuropsychological Assessment
The severity of symptoms for all patients was assessed using the Japanese version of the 17-item Hamilton Depression Rating Scale (HAM-D) and the Beck Depression Inventory–II (BDI-II) on the day of the PET scan (
19,
20). All participants performed three neuropsychological tests: the Trail Making Test, parts A and B, and the Symbol Digit Modalities Test (
21,
22). Trails A requires visual attention, and Trails B requires not only visual attention but also working memory. The Symbol Digit Modalities Test assesses executive functions such as complex scanning and visual tracking (
22,
23). Two patients failed to take the above-mentioned tests because of fatigue.
PET and MRI Acquisition
An ECAT EXACT HR+ (CTI-Siemens, United States) PET scanner system, which provides a 15.5-cm axial field of view, was used for all patients and healthy subjects with a head fixation device to minimize head movement. A 10-minute transmission scan was acquired after the emissions scan using a
68Ge-
68Ga source for subsequent attenuation correction. (
S,S)-[
18F]FMeNER-D
2 was synthesized by fluoromethylation of norethylreboxetine with
18F-bromofluoro-methane-d
2 (
24), yielding a radiochemical purity of higher than 95%. Emission data were acquired from 120 minutes to 180 minutes (10 minutes × 6 frames) in three-dimensional mode, following an intravenous bolus injection of (
S,S)-[
18F]FMeNER-D
2. Injected radioactivity averaged 186.2 MBq (SD=7.9) for patients and 188.0 MBq (SD=13.3) for healthy subjects, while specific radioactivity averaged 675.6 GBq/μmol (SD=388.7) for patients and 584.0 GBq/μmol (SD=394.5) for healthy subjects at the time of injection. Data were reconstructed by a filtered back-projection using the Hanning filter (6.3 mm, full width at half maximum).
MR images were acquired with a 3.0-T scanner, MAGNETOM Verio (SIEMENS, Germany). Three-dimensional volumetric acquisition of a T1-weighted gradient echo sequence produced a gapless series of thin sagittal sections (TE=1.95 ms, TR=2,300 ms, TI=900 ms, flip angle=9°, field of view=250 mm, acquisition matrix=256×256, slice thickness=1 mm, voxel size=1 mm×1 mm×1 mm). The MRI results revealed no apparent structural abnormalities.
Image Analysis
T
1-weighted MRIs were coregistered to the corresponding PET images using SPM8 (Wellcome Trust Centre for Neuroimaging, London). MR and PET images were then spatially normalized to Montreal Neurological Institute space (MNI 152 2 mm template) based on the transformation parameters estimated from the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) algorithm in SPM8 (
25). A template of regions of interest (Oxford thalamic connectivity atlas [
17]) was applied to the spatially normalized PET images to extract time activity curves for the subregions of the thalamus. The Oxford thalamic connectivity atlas is based on diffusion weighted imaging conducted by Behrens (
17). This atlas thus separates the thalamus into seven subregions that are anatomically connected with seven cerebral cortical regions. Manually drawn regions of interest were applied for the locus ceruleus according to Keren et al. (
26), as well as for the caudate, which was used as a reference region because it is almost devoid of norepinephrine transporter availability (
15,
27). Nondisplaceable binding potential (BP
ND) values were calculated using the area under the curve ratio (AUC) method (
13) from 120 minutes to 180 minutes using the following equation:
Statistical Analysis
Statistical analysis was performed using SPSS, version 22 (IBM, Armonk, N.Y.). Group differences in BPND values and neuropsychological performance between patients with major depressive disorder and healthy subjects were analyzed using independent sample t statistics (two-tailed) with the Bonferroni correction for multiple comparisons. The correlations between regional BPND values and neuropsychological performance, as well as between clinical variables (HAM-D total items, HAM-D insomnia items, HAM-D anxiety items, and BDI-II), were analyzed using age as a covariate followed by the Benjamini-Hochberg method for multiple comparisons using the false discovery rate controlling procedure with q*=0.05.
Results
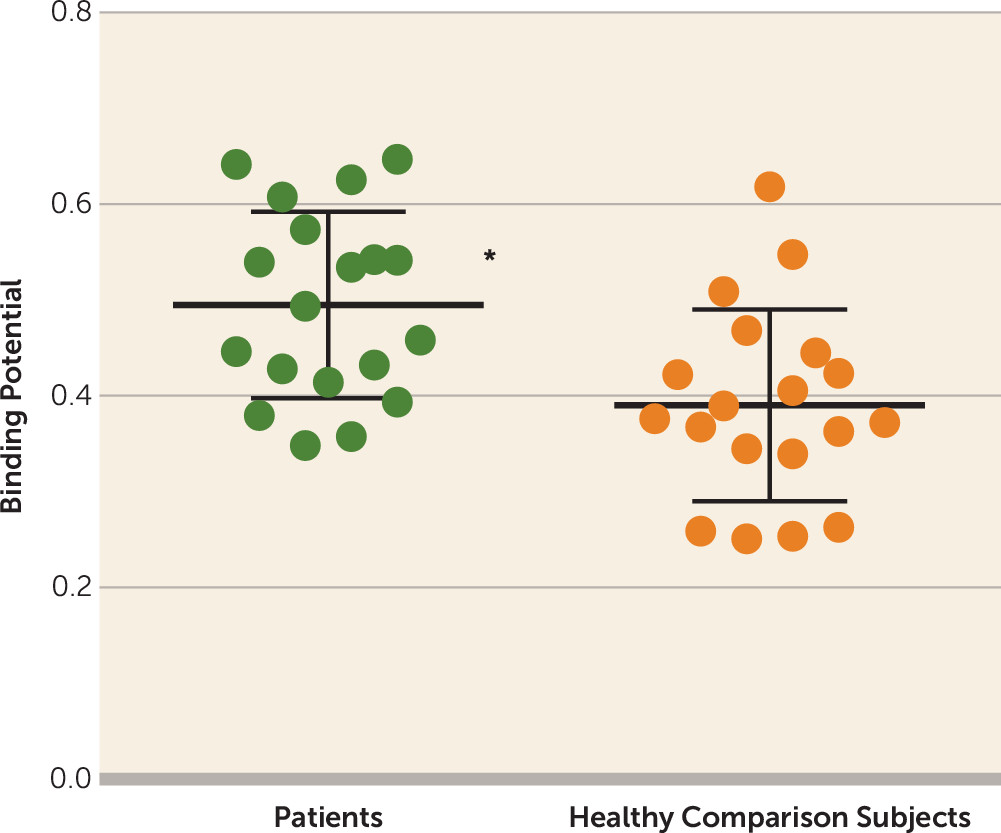
Table 1 presents clinical variables and neuropsychological performances. Relative to healthy comparison subjects, patients with major depressive disorder showed 29.0% higher BP
ND values in the thalamus (t=2.85, df=36, p=0.007; alpha=0.05/2, Bonferroni correction) and, in particular, 28.2% higher BP
ND values in the thalamic subregion connected to the prefrontal cortex (t=3.26, df=36, p=0.002; alpha level=0.05/7, Bonferroni correction) (
Figure 1 and
Table 2). BP
ND values of the locus ceruleus were not significantly different between patients with major depressive disorder and healthy subjects.
When controlling for age, BP
ND values were negatively correlated with reaction time in Trails A in the thalamus and locus ceruleus in patients (r=−0.70, p=0.003, and r=−0.56, p=0.023, respectively) (
Table 3). However, based on the Benjamini-Hochberg method for multiple comparisons, neuropsychological tests and the clinical variables did not correlate with the norepinephrine transporter BP
ND values in any regions.
Discussion
This study examined the in vivo norepinephrine transporter availability and symptoms in patients with major depressive disorder. Results showed that thalamic norepinephrine transporter availability in patients with major depressive disorder was significantly higher than that in healthy comparison subjects in the thalamus and in the thalamic subregion anatomically connected to the prefrontal cortex. We further found that elevated norepinephrine transporter availability in the locus ceruleus and thalamus in patients with major depressive disorder was positively correlated with visual search attention, as measured by Trails A.
Our finding regarding norepinephrine transporter availability in the thalamus is consistent with animal studies that have revealed elevated norepinephrine transporter in rat models of depression induced by chronic stress (
9,
10). Therefore, higher norepinephrine transporter availability in the thalamus in patients with depression may also indicate the involvement of stress-related response because the majority of our patients had melancholic depression, which is regarded as a stress response (
28).
At present, there is little evidence that helps explain the mechanism of this norepinephrine transporter regulation; however, there are several potential hypotheses. Dysfunctional norepinephrine systems are potential etiological factors in depression (
29), which may induce elevated norepinephrine transporter availability (
30). Hyperactive hypothalamic-pituitary-adrenal and norepinephrine systems have both been hypothesized as mechanisms involved in melancholic depression, which is the primary type of depression we see in our patients (
31,
32). Moreover, it should be noted that the norepinephrine system is not the only system involved in the altered norepinephrine transporter regulation. Other mechanisms, such as acetylcholine and GABA (
33), may also be involved in the regulation of the norepinephrine transporter (
34).
To our knowledge, there has been only one postmortem study investigating norepinephrine transporter in major depressive disorder (
3). Although that study reported a low norepinephrine transporter binding in the locus ceruleus in major depressive disorder, our PET study did not find a difference in BP
ND values of the same region between patients and healthy subjects. This finding does not necessarily mean that there is no change, but the limited spatial resolution of PET images makes it difficult to adequately detect alterations in small structures such as the locus ceruleus, which yields a high variability of BP
ND values in this region, as shown in
Table 2. In addition, we enrolled antidepressant-naive or antidepressant-free patients with early-onset depression, but the postmortem study included a wide range of patients who were taking antidepressant medication. Moreover, the locus ceruleus was the only region the earlier study investigated, whereas our study targeted the thalamus as well. Considering that chronic stress has been reported to increase norepinephrine transporter density in rat brain and antidepressants to lower it (
35), the effects of treatment and illness duration may influence the expression of norepinephrine transporter in major depressive disorder.
Regarding the neuropsychological tests, visual attention measured by Trails A was less affected, while working memory and executive function measured by Trails B and the Symbol Digit Modalities Test were lower in our patients (
Table 1), in accordance with previous findings of melancholic depressed patients (
36). In other words, visual attention is preserved among these neuropsychological functions, and the present findings suggest that the preserved visual attention measured by Trails A is associated with elevated norepinephrine transporter availability in depressed patients. A previous PET study demonstrated a positive correlation between vigilance and norepinephrine transporter availability in patients with PTSD (
12). Thus, elevated norepinephrine transporter availability may also be associated with enhanced attention and vigilance in our depressed patients. However, our results regarding the correlation did not withstand multiple comparisons. Thus, we should interpret the results carefully.
In the PET analysis with (
S,S)-[
18F]FMeNER-D
2, we used the AUC method. The validity of this method with (
S,S)-[
18F]FMeNER-D
2 PET for quantification of norepinephrine transporter availability has been reported previously (
13). When the AUC method is compared with kinetic modeling, BP
ND values in the thalamus obtained by the AUC method matched very well with the values obtained by kinetic modeling (r=0.88). Moreover, BP
ND values obtained by the AUC method are more reliable, with smaller intersubject variability, than those obtained by kinetic modeling in the thalamus. Thus, we chose the AUC method as a reliable method for the quantification of norepinephrine transporter availability using (
S,S)-[
18F]FMeNER-D
2 PET in this study.
Some limitations of the study should be addressed. First, our sample size was limited; after correction for multiple comparisons between the clinical variables and norepinephrine transporter availability, none of the correlations withstood significance testing. Thus, further studies with larger sample sizes are necessary to understand the role of norepinephrine transporter availability in patients with major depressive disorder. Moreover, because most of our patients experienced melancholic depression, we cannot conclude that all patients with major depressive disorder have high norepinephrine transporter availability. Second, by using (
S,S)-[
18F]FMeNER-D
2, we examined norepinephrine transporter availability only in the thalamus and the locus ceruleus. The norepinephrine transporter availability is insufficient for (
S,S)-[
18F]FMeNER-D
2 quantification in other brain regions, and the accumulated fluoride in bone makes it difficult to observe cortical regions through defluorination of this radioligand (
13). Thus, other techniques should be used to investigate norepinephrine transporter availability in different brain regions. Finally, there could be differences in norepinephrine availability in the caudate between patients and healthy subjects, and thus using the caudate as a reference may skew the results. An ideal reference region needs to be completely devoid of specific binding. However, this is often not the case for PET imaging. Regarding norepinephrine transporter availability, postmortem studies in nonhuman primates and humans have indicated that norepinephrine transporter availability in the caudate is very low (
27,
37). The very low specific binding in the caudate was confirmed by a PET occupancy study in rhesus monkeys (
38). After injection of a selective norepinephrine transporter inhibitor, no PET signal change was detected in the caudate compared with baseline readings, meaning the amount of specific binding in the caudate was significantly lower than that of the nonspecific binding of the PET ligand in the same region. Thus, the resulting BP
ND values in our study would be less affected by the group differences in specific binding in the caudate.
In conclusion, we found higher norepinephrine transporter availability in the thalamus and its subregions in patients with major depressive disorder. These findings implicate the potentially beneficial selection of antidepressants with affinity to the norepinephrine transporter in the treatment of major depressive disorder.