Generalization of conditioned fear is a basic, cross-species, associative-learning process whereby fear acquired to a conditioned stimulus (CS+), paired with an aversive unconditioned stimulus, transfers to safe stimuli resembling the CS+ (
1). Heightened levels of generalized conditioned fear have been adopted as a core feature of trauma-related psychopathology (
2), and DSM-5 criteria for posttraumatic stress disorder (PTSD) include heightened distress to situations “resembling” aspects of the trauma. The pathogenic contribution of conditioned generalization to PTSD follows from the undue proliferation of trauma cues in an individual’s posttrauma environment that then increases and/or sustains PTSD symptoms.
In lower mammals, lesions of either the hippocampus (
6,
7) or the cortical inputs to the hippocampus (the postrhinal or perirhinal cortex) (
8) increase generalization of fear from CS+ to resembling conditioned safety cues (CS–). Findings from these studies suggest that the hippocampus is necessary for successful discrimination of CS+ from CS–, potentially attributable to hippocampally mediated pattern separation found in rodents (
9) and humans (
10) through which brain representations of resembling, yet distinct, sensory experiences are discriminated. Consistently, our previous functional MRI (fMRI) results demonstrate robust gradients of generalization in the human hippocampus. Specifically, activations in the left and right ventral hippocampus were strongest to GS most distinguishable from CS+ (stimuli for which pattern separation is most appropriate), with levels decreasing bilaterally as the presented stimulus became more similar to CS+ (
11). These results are consistent with the proposed role of hippocampally mediated pattern separation in human conditioned fear generalization.
Additional candidate brain substrates of human generalization derive from the long-observed finding that GS elicit the same response evoked by CS+, with gradual declines as the GS differentiate from CS+ (
12). Thus, fear-related brain activations to CS+ found repeatedly in the amygdala (
13), anterior insula (
13,
14), dorsomedial prefrontal cortex (
13–
15), dorsal anterior cingulate (
14), inferior parietal lobule (
14), and dorsolateral prefrontal cortex (
16) are predicted to decrease as the presented GS diverge from CS+. Conversely, safety-related activations to CS– repeatedly found by human neuroimaging studies in the ventromedial prefrontal cortex (
17), hippocampus (
17), and precuneus (
17) are predicted to gradually increase as the GS become less similar to CS+. Results from recent fMRI studies of fear generalization are largely consistent with these predictions and find gradually decreasing activations in the anterior insula (
11,
16,
18–
20), dorsomedial prefrontal cortex (
11,
18,
19), inferior parietal lobule (
11), and dorsolateral prefrontal cortex (
11) (downward gradients) and gradually increasing activations in the ventromedial prefrontal cortex (
11,
18–
20), hippocampus (
11,
20), and precuneus (
11) (upward gradients) as the presented GS differentiate from CS+. Furthermore, several such activations have been linked to the hippocampus, as functional connectivity studies reveal stronger connectivity between the ventral hippocampus and brain areas associated with fear excitation (amygdala, anterior insula) to stimuli with more, as opposed to less, resemblance to CS+, as well as stronger connectivity between the ventral hippocampus and brain areas associated with fear inhibition (ventromedial prefrontal cortex, precuneus) to stimuli with less, as opposed to more, resemblance to CS+ (
11). These connectivity findings are consistent with the proposal that exposure to GS elicits hippocampal activation of neural substrates of either fear excitation or fear inhibition, depending on the degree of similarity between the GS and CS+ (
11).
Discussion
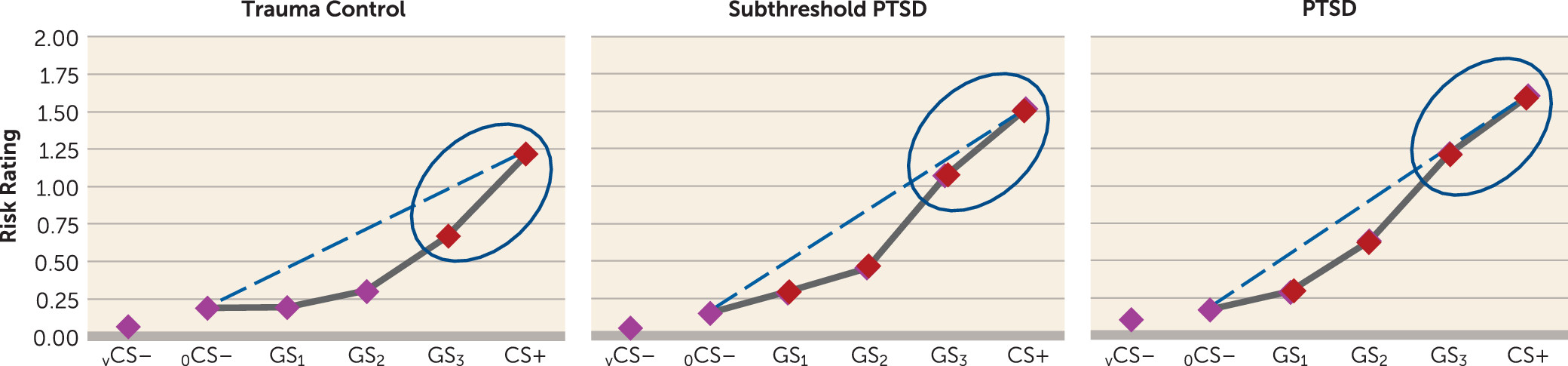
The present findings demonstrate overgeneralized fear to safe stimuli resembling conditioned danger cues in PTSD and elucidate brain substrates of this abnormality. Specifically, behavioral gradients of generalized conditioned fear in trauma control subjects formed steep quadratic declines, the gradient shape repeatedly found in intact animals (
3) and healthy humans (
4). By contrast, individuals with PTSD and to some degree those with subthreshold PTSD displayed behavioral gradients characterized by more gradual linear declines, reminiscent of gradient shapes found in panic (
26) or generalized anxiety disorder (
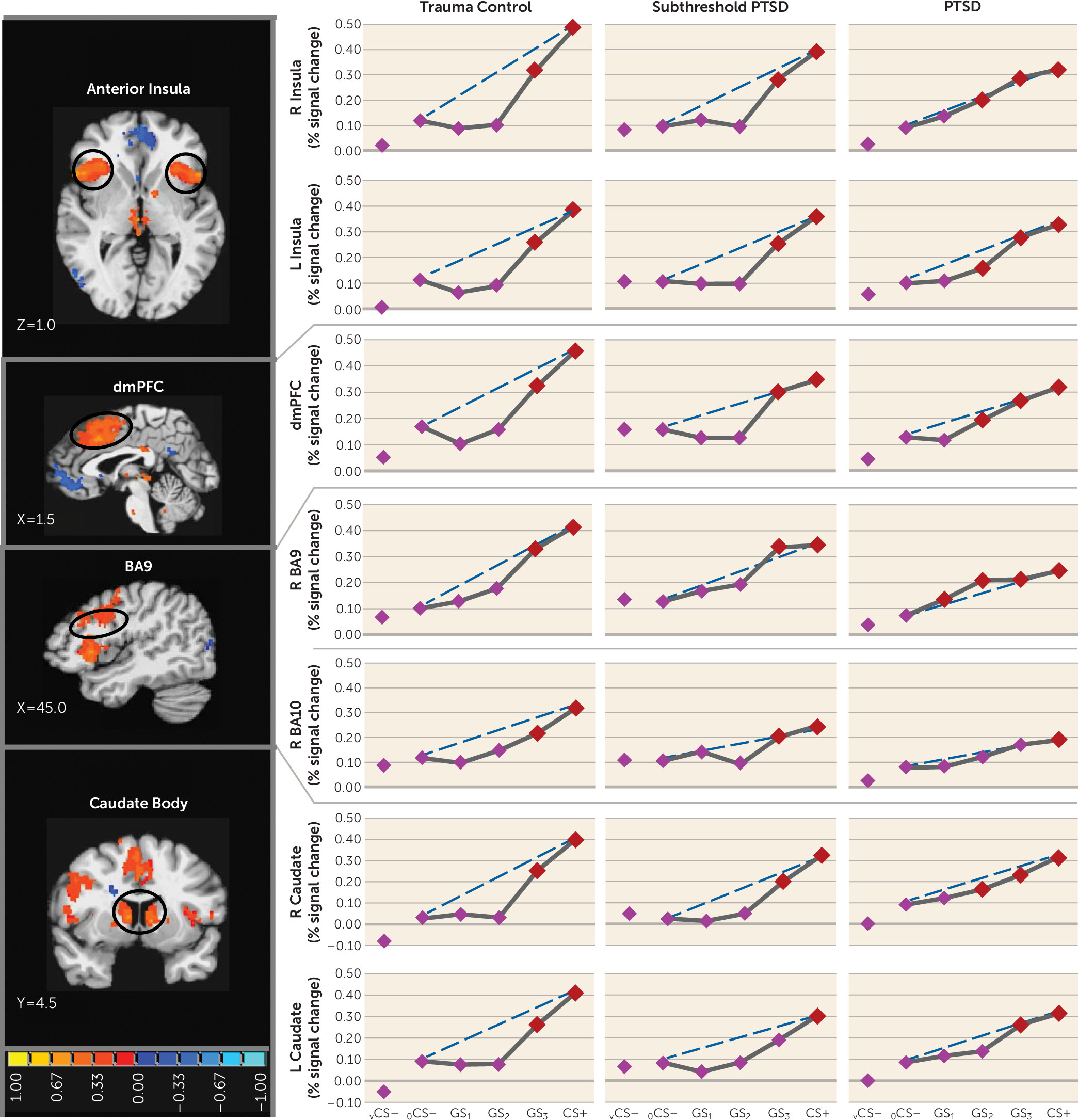
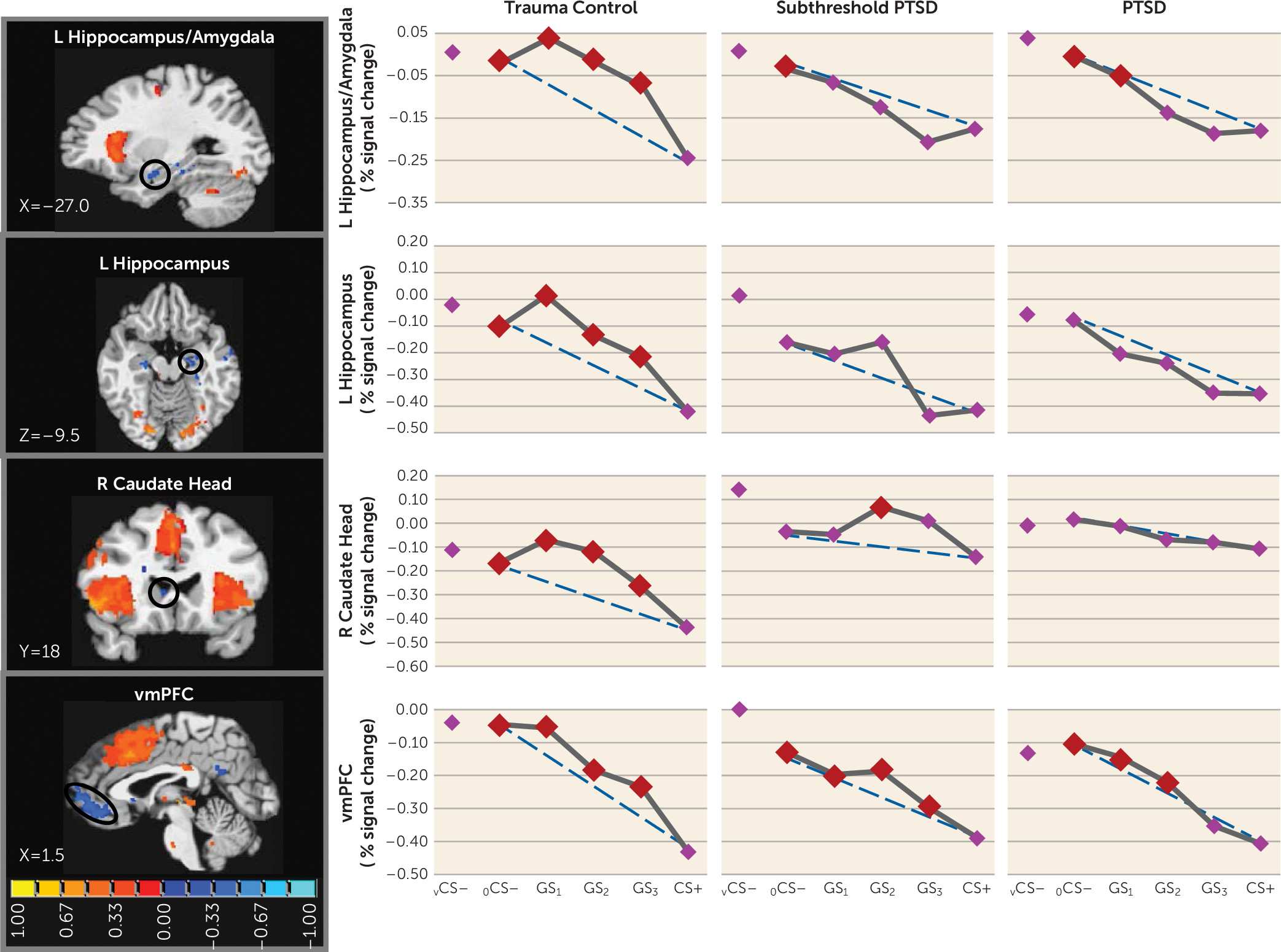
25) and indicating heightened persistence (generalization) of fear as presented stimuli differentiate from CS+. Neural results consistently mirrored this behavioral pattern, with most functional regions of interest displaying steeper, quadratic generalization gradients in trauma control subjects but more gradual linear gradients in PTSD subjects and, to some degree, in subthreshold PTSD individuals, indicative of overgeneralization. This pattern of group differences was found in brain areas coding for both positive (left anterior insula, right anterior insula, dorsomedial prefrontal cortex, dorsolateral prefrontal cortex [BA9], left and right caudate, right inferior parietal lobule) and negative generalization gradients (left ventral hippocampus/amygdala, and right caudate head).
Many of the aforementioned neural substrates of overgeneralization in PTSD have been previously shown to subserve human fear generalization (anterior insula [
11,
16,
18–
20], ventromedial prefrontal cortex [
11,
18–
20], ventral hippocampus [
11,
20], dorsomedial prefrontal cortex [
11,
18,
19], inferior parietal lobule [
11], dorsolateral prefrontal cortex [
11]), and many are constituents of a proposed neurobiology of generalization (
11). Central to the model is discrimination of CS+ from resembling stimuli via hippocampally mediated pattern separation. When faced with a new stimulus event (i.e., a generalization stimulus) that resembles a past event stored in memory (i.e., CS+), the hippocampus is thought to perform a same-different determination between cortical representations of current and past events (
27,
28). With decreasing representational overlap, the hippocampus increasingly differentiates cortical representations of current (GS) and past events (CS+) through pattern separation (
9). When the past event is fear related, such pattern separation culminates in hippocampal activation of brain regions associated with fear inhibition (e.g., the ventromedial prefrontal cortex) (
11). As in past generalization work (
11), the present findings document ventral hippocampus activations falling along negative gradients, with the strongest responses to stimuli with the least schematic match to CS+ (i.e., vCS–, oCS–), or in other words, to stimuli most likely to elicit pattern separation. Furthermore, past and current hippocampal activations are least strong to CS+ and gradually strengthen with increasing CS+ differentiation (i.e., as pattern separation becomes more appropriate). Importantly, these negative generalization gradients in the ventral hippocampus were steep or quadratic in trauma control subjects but were gradual or linear in PTSD and subthreshold PTSD subjects, with significant ventral hippocampus increases (relative to CS+) requiring at least three degrees of CS+ differentiation in PTSD and subthreshold PTSD subjects (GS
1–GS
3) but only one degree in trauma control subjects (GS
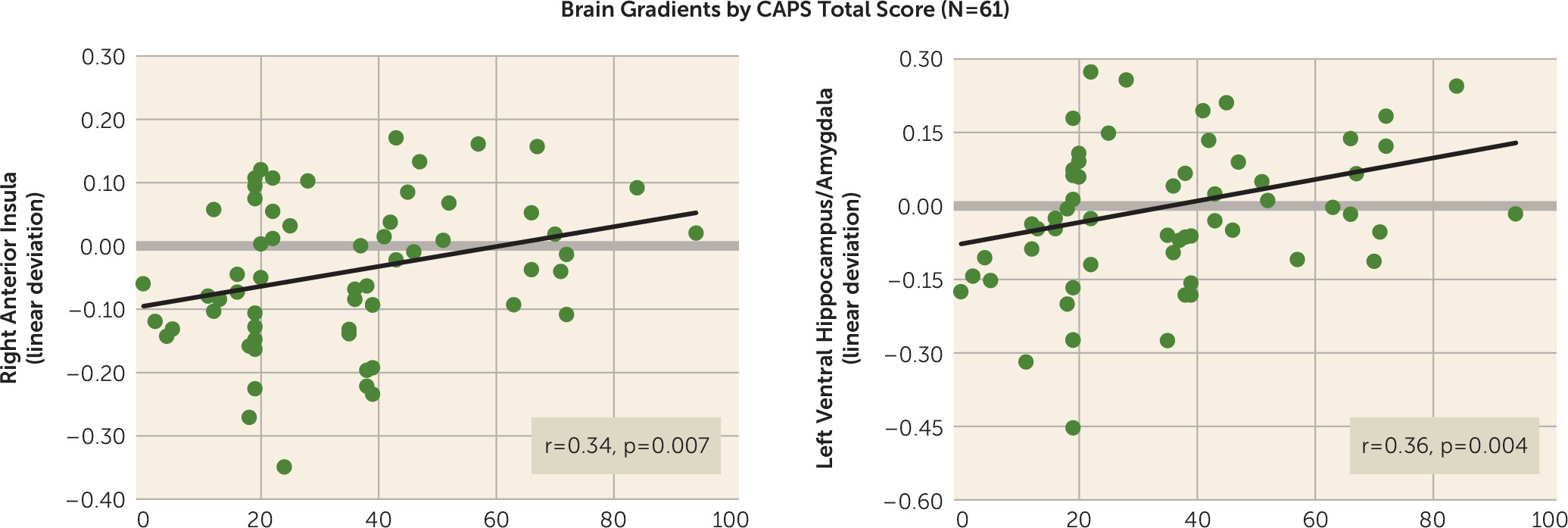
3). Such results suggest deficits in hippocampally mediated pattern separation in PTSD through which subjects with PTSD or subthreshold PTSD require more CS+ dissimilarity before generalization is blocked by such pattern separation. Further supporting the relationship between PTSD and ventral hippocampus gradient shape was the correlation between CAPS scores and steepness of ventral hippocampus gradients, implicating levels of generalization in the ventral hippocampus as a promising brain marker of PTSD symptom severity.
The less steep ventral hippocampus gradients in PTSD described above were expected to be accompanied by less steep ventromedial prefrontal cortex gradients in PTSD, given the proposed positive relationship between hippocampally mediated pattern separation and activations in the ventromedial prefrontal cortex (
11). Although negative gradients in the ventral hippocampus were mirrored by similarly shaped negative gradients in the ventromedial prefrontal cortex, no group differences in the shape of the ventromedial prefrontal cortex emerged. Furthermore, functional connectivity between the ventral hippocampus and the ventromedial prefrontal cortex differed across groups but in a direction opposite to what was predicted. PTSD showed stronger coupling of the ventral hippocampus and ventromedial prefrontal cortex during GS compared with vCS–, suggesting that hippocampally mediated pattern separation actually activated fear inhibition mediated by the ventromedial prefrontal cortex during GS (relative to vCS–) to a greater extent in subjects with PTSD, which should culminate in less fear to GS in PTSD. However, this connectivity finding is consistent with overgeneralization in PTSD if it is interpreted as being driven by overly strong fear reactivity to GS in PTSD, which then necessitates more connectivity between the ventral hippocampus and ventromedial prefrontal cortex to (perhaps unsuccessfully) attempt inhibition of the heightened fear to GS.
In addition to hippocampal activation of areas of fear inhibition in the brain (ventromedial prefrontal cortex) commensurate with the dissimilarity of a given generalization stimulus from CS+, our generalization model proposes separate hippocampal areas (i.e., the CA3 subregion) that activate fear-excitation areas commensurate with the degree of similarity (
29). That is, greater schematic matches between a given generalization stimulus and the previously encountered CS+ should increase the likelihood of hippocampally mediated pattern completion (
30), resulting in activation of the total pattern of brain activity subserving CS+, including fear-excitation brain areas. Although no observed hippocampal activation showed positive gradients reflective of pattern completion, GS with stronger schematic matches to CS+ resulted in stronger activation of such brain areas associated with fear excitation as the left and right anterior insula (
13,
14), dorsomedial prefrontal cortex (
13–
15), and inferior parietal lobule (
14). Importantly, generalization gradients in these fear-excitation areas were consistently less steep in PTSD subjects relative to trauma control subjects, implicating these regions as neural substrates of PTSD-related overgeneralization. Further supporting this assertion were the observed correlations between PTSD symptom severity and gradient steepness in the right anterior insula and approaching significance in the dorsomedial prefrontal cortex. Finally, psychophysiological interaction results indicated a ventral hippocampus contribution to group effects in these and other fear-excitation brain loci, with PTSD subjects showing greater functional connectivity than trauma control subjects between the ventral hippocampus and anterior insula, dorsomedial prefrontal cortex, inferior parietal lobule, and amygdala during GS compared with vCS–. Such results provide some evidence for the predicted increases in hippocampal connectivity to fear-related brain areas during stimuli resembling CS+.
Our results also link PTSD-related overgeneralization to brain regions not included in the model: the dorsolateral prefrontal cortex (BA9) and caudate nucleus. Because lateral portions of BA9 have been shown to code for cognitive control of emotion (
31), BA9 activations may reflect subjects’ attempts to cognitively down-regulate fear evoked by CS+ or GS. Thus, greater generalization instantiated in BA9 among those with PTSD may indicate a more persistent need for cognitive control of fear as the presented stimulus differentiates from CS+. The caudate body has been found to subserve motivated behavior including anxiety-driven avoidance (
32,
33). Although behavioral avoidance of shock was not possible in this study, subjects may have felt the impulse to avoid when exposed to CS+ or resembling GS. Thus, group effects in the caudate body may reflect greater generalization of the impulse to avoid among those with PTSD.
One unexpected finding was the absence of a correlation between PTSD symptom severity and behavioral generalization. Although this correlation was in the expected direction, it was not significant. Thus, while both PTSD and subthreshold PTSD groups showed heightened behavioral generalization, when measuring PTSD symptoms continuously, the relation between PTSD symptoms and generalization was not significant. One possibility for this occurrence is that our study was sufficiently powered to identify group differences in behavioral generalization but was underpowered for assessing relations between continuous symptom severity and behavioral generalization.
Treatment Implications
The link between PTSD and overgeneralization prescribes a therapeutic focus on reducing fear to benign stimulus events resembling features of the traumatic encounter, in addition to the actual features of the trauma. Specifically, exposure treatments might focus on reducing fear reactivity to both stimuli associated with the trauma and stimuli approximating those associated with the trauma. This could be done through in vivo systematic desensitization using a hierarchy of feared stimuli, with exposures to stimuli resembling the feared stimulus added at each level of the hierarchy. In addition, patients could undergo discrimination training, whereby they learn to differentiate trauma cues indicative of genuine danger from benign cues with inconsequential resemblance to the trauma.
For patients with refractory symptoms, this “generalization-focused” exposure could potentially be enhanced pharmacologically in at least one of two ways. First, studies in humans and lower mammals suggest that conditioning-dependent retuning of sensory representations of the conditioned danger cue toward resembling stimuli leads to overgeneralization by rendering perceptual discrimination of the danger cue from its approximations more difficult (
34,
35). This conditioning-dependent effect has been tightly linked to the cholinergic system (
35,
36). Consequently, medications with anticholinergic properties (e.g., scopolamine) given just prior to sessions of generalization-focused exposure therapy may enhance the therapeutic efficacy of this exposure treatment by facilitating improved sensory discrimination of feared stimuli from their approximations during discrimination training.
Second, findings in lower mammals demonstrate that pretraining administration of
d-cycloserine, a partial agonist at the
N-methyl-
d-aspartate receptor, reduces generalization of Pavlovian fear by enhancing an organism’s ability to discriminate conditioned danger cues from resembling conditioned safety cues (
37,
38). Given findings that
d-cycloserine strengthens acquisition of aversive conditioning (
39),
d-cycloserine may enhance conditioned discrimination by strengthening the accuracy of learning, with a resulting decrease in generalization errors. Thus,
d-cycloserine has the potential to strengthen the corrective learning acquired during generalization-focused exposure therapy (i.e., reduced generalization) by both enhancing fear reduction to benign stimuli resembling feared stimuli and strengthening discrimination learning. Although these novel pharmacologically enhanced exposure treatments are, at this point, speculative, the feasibility of these treatment approaches is supported by the fact that both scopolamine and
d-cycloserine are safe for human use.
In conclusion, these results represent the first demonstration of overgeneralized fear conditioning in PTSD using generalization gradients, the gold standard for systematic assessments of stimulus generalization. Patterns of overgeneralization in PTSD were highly consistent across behavioral measures and a host of generalization-coding neural activations (the left and right anterior insula, dorsomedial prefrontal cortex, right dorsolateral prefrontal cortex, left ventral hippocampus, left and right caudate body, right caudate head). Specifically, behavioral and neural gradients in PTSD subjects relative to trauma control subjects were uniformly characterized by more gradual linear generalization gradients indicative of greater persistence of fear as the presented stimulus differentiated from CS+. Furthermore, PTSD symptom severity was positively correlated with strength of generalization at two of these neural loci (the right anterior insula and left ventral hippocampus). The present results validate the applied translational paradigm for behavioral and neural assessments of trauma-related abnormalities in conditioned fear generalization and support the development of novel interventions for PTSD that aim to reduce levels of generalized conditioned fear. Longitudinal work is needed to determine whether PTSD-related abnormalities in generalization predate the onset of PTSD and contribute toward its development or reflect ongoing disease processes of the disorder.