Microglia are a key player in the immune surveillance system of the CNS, where they act as resident macrophages and are the first responders to various types of brain insult (
1). As part of the brain inflammatory response, microglia are transformed from a “sentry” state into an “active” state (
1,
2). In this process, the microglia change their morphology from branched to globular, with a large increase in cell volume, while migrating to and engulfing the site of insult (
3,
4). Activated microglia (for a review, see reference
5) express elevated levels of a protein in their mitochondria known as translocator protein 18 kDa (TSPO) (
6). Because TSPO is overexpressed in response to neuroinflammation as compared with normal tissue, it represents an important imaging target for microglial activation.
Over the years, research has focused on the immune response and neuroinflammation as likely contributors to schizophrenia (
7–
14). Genome-wide association studies (GWAS) have reported links between schizophrenia and genetic variants in the major histocompatibility complex (MHC) genes, supporting the involvement of the immune system in the pathogenesis of schizophrenia (
15,
16). Several lines of research support these GWAS findings: 1) in animal models, an induced maternal immune response (administration of synthetic viral analogue polyriboinosinic-polyribocytidillic [poly I:C] acid [
17]) results in behavioral, neurochemical (
18–
20), and structural changes (
17) in the brains of the offspring that resemble the immune alterations implicated in schizophrenia; 2) prenatal exposure to a variety of infections has been linked with an increased risk of developing schizophrenia (
21–
25); and 3) elevated plasma levels of proinflammatory cytokines in schizophrenia provide evidence for the involvement of peripheral inflammatory response (for a review, see reference
26). However, postmortem studies examining microglia abnormality in schizophrenia are inconclusive; increased microglia activation (
27), decreased microglial activation (
28), and no difference in microglial activation (
29) have been reported. Given the limitations of postmortem studies, positron emission tomography (PET) with TSPO radioligands provides an opportunity to study microglial activation in schizophrenia in vivo.
To date, of five PET studies that have investigated this question in schizophrenia patients on antipsychotic treatment (
30–
34), three have reported results in line with increased microglial activation in schizophrenia (
30–
32). The first two of these (
30,
31) showed increased binding potential of [
11C]PK11195 in schizophrenia compared with healthy volunteers in total gray matter and in the hippocampus, respectively. However, imaging studies using this early radiotracer were hindered by its recognized methodological limitations (
35). Using the new generation of TSPO radiotracer [
11C]DAA1106, Takano et al. (
33) found no difference in binding between schizophrenia patients who had received chronic treatment (N=14) and healthy volunteers (N=14), but they reported significant positive associations between tracer binding, duration of illness, and positive psychotic symptoms. Our recent study evaluating microglial activation in 18 treated patients with schizophrenia (
34) using [
18F]FEPPA and controlling for TSPO genotype (rs6971 polymorphism) found neither a difference between groups nor any correlation between tracer binding and symptom severity, cognition, or duration of illness. While a recent study using another second-generation TSPO radioligand showed higher [
11C]PBR28 distribution volume ratios (DVRs—a different outcome measure reflecting change relative to another brain region) in total gray matter, frontal lobes, and temporal lobes of schizophrenia patients relative to comparison subjects (
32), no significant differences were found between the groups when using the validated [
11C]PBR28 outcome measure total distribution volume (V
T) (
36).
Recent studies with new-generation PET radioligands targeting TSPO report significant intersubject variability in binding. On the basis of analyses performed in brain tissue and platelets, three distinct levels of binding affinity have been noted, with participants grouped as high-affinity binders, low-affinity binders, and mixed-affinity binders (
37–
39). A polymorphism in exon 4 of the TSPO gene (rs6971) has been implicated in this differential binding affinity (
40), suggesting that rs6971 gene polymorphism needs to be considered in the quantification of second-generation TSPO radioligands, including [
18F]FEPPA (
41). Using this methodology, recent PET studies in Alzheimer’s disease and major depressive episode using [
18F]FEPPA have demonstrated the ability of this radiotracer to quantify microglia activation in humans (
42,
43).
In the present study, we used the validated [
18F]FEPPA V
T to examine microglial activation in untreated patients in first-episode psychosis with either minimal (less than 4 weeks) or no lifetime exposure to antipsychotics. We explored the association between microglial activation and severity of psychopathology as well as neuropsychological deficits, given reported positive associations between microglial activation and severity of symptoms in schizophrenia (
32,
33). We also explored DVRs as a pseudo-reference region method—in this case, the ratio of [
18F]FEPPA V
T in the region of interest to V
T in cerebellum, gray matter, or whole brain (
44).
Discussion
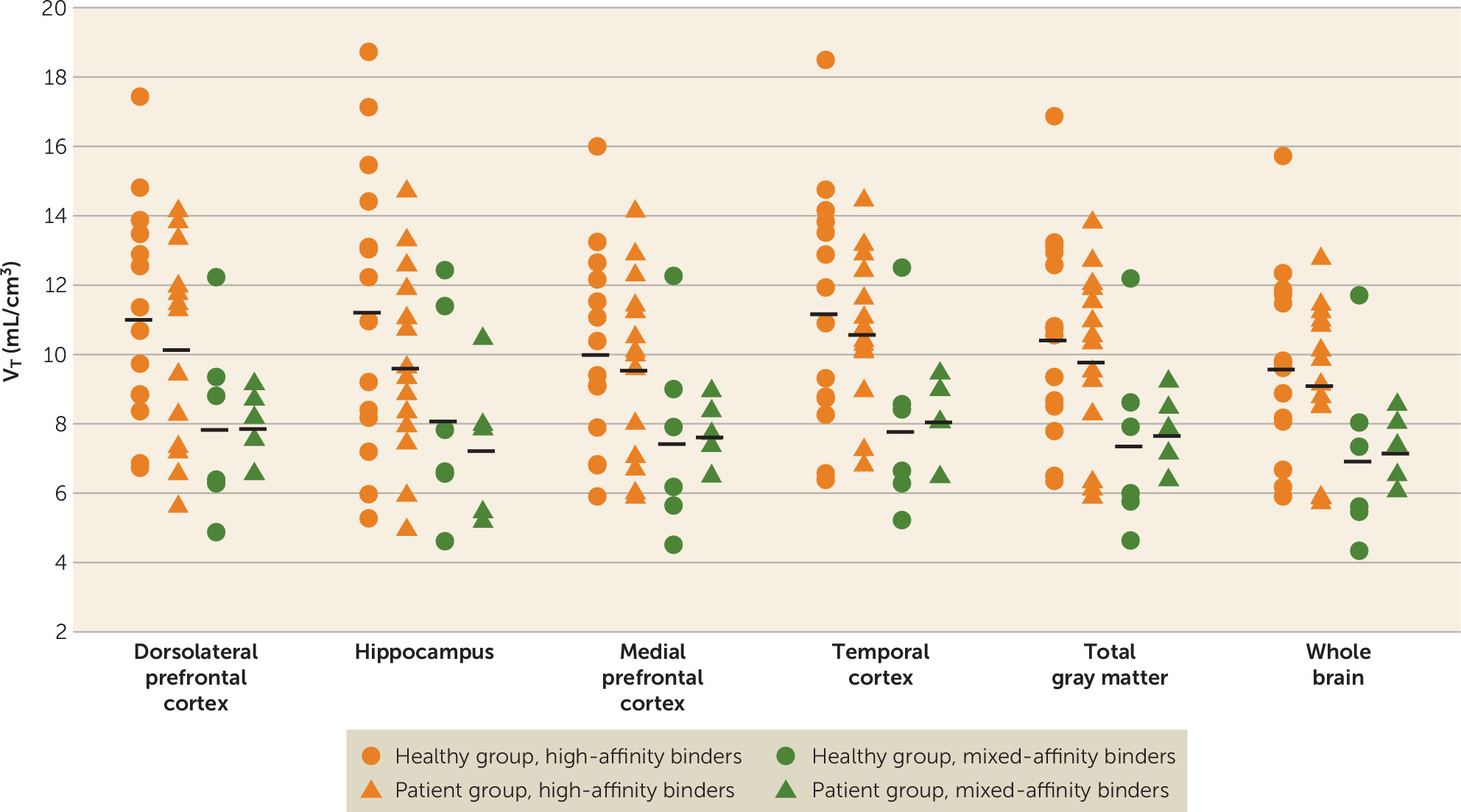
To the best of our knowledge, this is the first PET study to evaluate microglial activation using a second-generation TSPO radioligand in untreated, mostly antipsychotic-naive patients in first-episode psychosis.
Early PET studies using [
11C]PK11195 reported increased tracer binding in the hippocampus of patients with schizophrenia (
30) and in the total gray matter of patients with recent-onset schizophrenia (
31). Based on previous reports (
27,
30) and a postmortem study using second-generation [
3H]PBR28 (
27), we expected significantly higher [
18F]FEPPA binding in the hippocampus and dorsolateral prefrontal cortex of patients with first-episode psychosis compared with healthy volunteers. In the present study, despite the use of a second-generation TSPO radioligand and scanning all untreated (mostly antipsychotic naive) first-episode patients in a high-resolution research tomography scanner, we observed no significant differences in [
18F]FEPPA binding between groups. While not statistically significant, [
18F]FEPPA uptake was higher in the healthy group than in the patient group in the hippocampus (13.4% higher) and the dorsolateral prefrontal cortex (6% higher). Additionally, we explored [
18F]FEPPA binding in the medial prefrontal cortex, the temporal cortex, total gray matter, and whole brain and obtained similar results. Nevertheless, our results are in line with other investigations using second-generation TSPO ligands, such as a study that found no differences in [
11C]DAA1106 binding in chronic schizophrenia patients (
33) and our previous study using [
18F]FEPPA in patients with schizophrenia who had received chronic treatment (
34). The present study therefore confirms these findings in a larger sample and in untreated, mostly first-episode patients.
While V
T is the gold standard to quantify [
18F]FEPPA binding (
49), because there is no reference region available, DVR has been proposed as an alternative measure (
44). Thus, we also present regional DVR values showing no significant differences between the patient and healthy groups. This finding is inconsistent with the recent study (
32) using the second-generation TSPO radioligand [
11C]PBR28, which showed higher microglial activation indexed as DVRs in total gray matter and the frontal and temporal lobes of schizophrenia patients compared with healthy volunteers. It should be noted that, using the gold-standard two-tissue compartment model with [
11C]PBR28 V
T as the outcome measure, the authors of that study did not find any significant difference between schizophrenia patients and healthy volunteers, in line with the present findings and with other studies using second-generation radioligands (
33,
34).
The variability of VT in this study, as with other second-generation TSPO ligands, was relatively high even after controlling for the effect of rs6971 polymorphism on binding affinity. However, sample size calculations using the present data suggest that to detect group effects (i.e., higher microglial activation in healthy volunteers than in first-episode patients) in the dorsolateral prefrontal cortex (the observed effect size was 0.22) or the hippocampus (the observed effect was 0.42), we would need 316 or 89 subjects per group, respectively, for a significance level of 0.05 (two-tailed) and 80% power in each brain region.
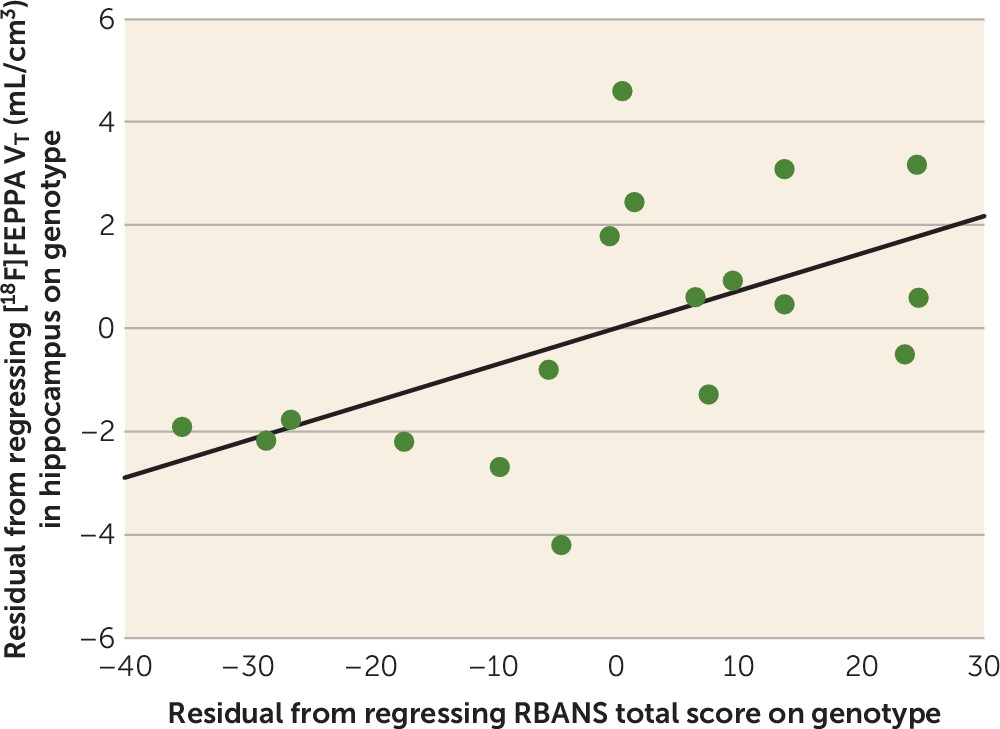
In patients with first-episode psychosis, we found a trend toward positive correlation between [
18F]FEPPA V
T in the hippocampus and RBANS total score, suggesting that higher microglial activation in the hippocampus is associated with better cognitive function. Similar trends were present when exploring associations between [
18F]FEPPA V
T and clinical measures in first-episode psychosis. Although this may be explained by multiple beneficial roles that microglia play in the CNS (
52), further studies will clarify the link between psychopathology and neuroinflammation in psychosis.
The results of this study should be interpreted with several limitations in mind. First, a significantly larger amount of [
18F]FEPPA was administered to the patient group, on average, than to the healthy group. However, this did not affect our results, as there were no differences between groups in the specific activity or the mass injected. Moreover, we did not find any significant correlations between the amount injected and binding of the radiotracer in any of the regions of interest. Second, studies have shown that microglia are not the only cells that express TSPO, and thus it is possible that a portion of the signal of [
18F]FEPPA binding comes from astrocytes (
53). Nevertheless, this would not have affected the overall conclusions of our study. TSPO is under neurohormonal control, and there are suggestions that other factors, such as stress and anxiety, can affect TSPO expression (
54,
55). Since these factors are commonly seen in psychotic patients, they should be taken into consideration in future studies. We explored a subsample of patients (N=5) for whom a stress scale was available and found a positive association between the state subscale of the State-Trait Anxiety Inventory and [
18F]FEPPA binding in the hippocampus (r=0.978, p=0.022) (see Figure S3 in the
data supplement). Third, some research groups have reported differences in the arterial input function between schizophrenia patients and comparison subjects (
32). While the robust outcome measure V
T is inherently designed to be unbiased under such circumstances, using repeated-measures mixed-model analysis, we empirically compared the arterial input function between patients and healthy volunteers at four different time intervals (0–30 minutes, 30–60 minutes, 60–90 minutes, and 90–120 minutes of the PET scan) and found no group differences. Finally, in neurochemical brain imaging studies, relatively small sample sizes constantly represent a potential limitation; however, to our knowledge, this is the largest PET study to date investigating microglial activation in first-episode psychosis, particularly in mostly antipsychotic-naive patients. While similar sample sizes were sufficient to test a difference between patients with Alzheimer’s disease and patients with major depressive episode compared with healthy volunteers using the same radioligand (
42,
43), it is possible that microglial activation may have a smaller magnitude in first-episode psychosis and would thus necessitate a larger sample size (as described above).
In conclusion, we found no evidence of increased microglial activation as indexed with [18F]FEPPA binding in the dorsolateral prefrontal cortex and hippocampus in patients with first-episode psychosis compared with healthy volunteers. The lack of significant between-group differences in [18F]FEPPA VT suggests that microglial activation is not present in first-episode psychosis. Neuroinflammatory processes may take place earlier in the course of schizophrenia, such as during the clinical high-risk state of psychosis, or may be present only in a subpopulation of patients.