Exposure to early-life stress robustly predicts a wide range of negative outcomes throughout the lifespan (
1–
3). Intimate partner violence (IPV)—a pattern of behavior in which one intimate partner threatens, intimidates, isolates, coerces, or uses emotional, sexual, or economic abuse to control the other partner—is a prevalent form of early-life stress (
1). There is, however, marked variability in outcomes following early-life stress, raising questions about individual differences in genetic vulnerability to such adverse environments. Identifying factors contributing to negative outcomes following early-life stress is of great clinical importance and may inform interventions to enhance resilience.
Several genetic polymorphisms in genes involved in the stress response, mainly in the hypothalamic-pituitary-adrenal (HPA) axis, are thought to moderate the impact of adversity on negative outcomes (
4). Among the genes more consistently found to moderate the relationship between early adversity and negative outcomes is FK506 binding protein 5 (
FKBP5).
FKBP5 plays an important role in the HPA axis through regulation of the glucocorticoid receptor complex.
FKBP5 provides an ultra-short feedback of glucocorticoid receptor sensitivity by being transcriptionally activated by the glucocorticoid receptor and subsequently limiting receptor activity via protein-protein interactions, with systemic consequences for the HPA axis’s set point (for a review, see reference
5). This molecular feedback regulation is moderated by common genetic variations. Four single-nucleotide polymorphisms (SNPs) (rs9296158, rs3800373, rs1360780, and rs9470080) within a functional haplotype in the
FKBP5 locus have received the most attention. Molecular studies have shown that the CATT haplotype leads to an enhanced induction of FKBP5 expression with exposure to glucocorticoids by altering the three-dimensional structure of the locus (
6–
10). Increased FKBP5 mRNA expression in postmortem brains of patients with psychiatric disorders and animal studies using transgenic, viral, and pharmacological tools support the hypothesis that increased expression of FKBP5, especially in the hippocampus and the amygdala, leads to increased anxiety and decreased stress coping (for a review, see reference
5). This may suggest that increased FKBP5 expression driven by genetic and environmental factors could contribute to psychiatric disorders. Indeed, this high-expression haplotype, when combined with exposure to early adversity, predicts a host of psychiatric symptoms and traits, including depression, posttraumatic stress disorder, psychosis, and suicide attempts, as well as impaired learning and memory in adulthood (
5). Given these broad observed associations, one could hypothesize that these diverse outcomes in adulthood may be driven by similar mechanisms early in life, with a common risk trajectory in childhood, followed by divergent symptom presentations later in life. In support of this notion, effects of the interaction of
FKBP5 and early-life stress on known early risk factors for the development of psychiatric symptoms have been documented in children, ranging from greater stress-induced cortisol reactivity in infancy to heightened risk for anxiety, depression, substance use, and dissociation during adolescence (for a review, see reference
5).
Expanding on previous findings, in this study we investigated interrelated aspects of self-regulation as a potential common pathway through which the interaction of early-life stress and
FKBP5 genotype increases risk for diverse negative outcomes, including psychopathology and poor academic achievement, in a longitudinal data set. We define self-regulation as a bidirectional system composed of cognitive, emotional, and physiological components that are hierarchically organized and reciprocally integrated. In its mature form, self-regulation is characterized by reflective, “top-down” control of behavior, in which executive function and the volitional control of attention can regulate emotional and physiological arousal in ways that promote engagement in goal-directed actions. Self-regulation, however, is also characterized by reactive, “bottom-up” control of behavior, in which levels of physiological arousal associated with the stress response and emotional reactivity can support or undercut the brain’s capacity for volitional top-down control (
11). Mechanistically, these associations are driven by hormones (glucocorticoids and monoamines) that, at moderate levels, increase neural activity in the prefrontal cortex but at high levels shut down activity in the prefrontal cortex and increase activity in the amygdala. Thus, the effective regulation of emotion and the physiological response to stress during infancy and toddlerhood sets the stage for the development of higher-order self-regulation abilities, such as executive function (
12,
13). Early emotion regulation and executive function abilities have been associated with a broad range of outcomes later in life, including mental health outcomes, school readiness, and academic achievement (
12).
From infancy onward, self-regulation develops through the interplay between individual characteristics and environment via the stress hormone system, with consequences for brain development (
13). Animal models have demonstrated that increased corticosteroid levels in response to early-life stress alter gene expression and induce structural and connectivity changes in brain areas involved in the regulation of stress response physiology (
14–
17), which modulates the neuroendocrine systems that underlie behavioral responses to stress. Variation in neural connectivity influences interindividual variation in stress physiology (
17). Through this interplay, early-life stress can have wide-ranging effects on aspects of social-emotional and cognitive development as the self-regulation system adapts to conditions of high threat and vigilance.
Building on this empirical and theoretical basis, we hypothesized that the combined effects of early-life stress and FKBP5 are expected to influence stress-induced cortisol reactivity and phenotypes associated with impaired self-regulation. To test this hypothesis, we investigated the interaction between IPV exposure over the child’s first 2 years and functional FKBP5 haplotypes in shaping self-regulation abilities across developmental stages. The self-regulation abilities examined in this study were prolonged stress-induced cortisol reactivity and fear-elicited emotional reactivity in infancy and toddlerhood, executive function in early childhood, and emotional and behavioral difficulties and reading and math abilities across the primary school grades (see Figure S1 in the online supplement for a schematic diagram).
Methods
Participants
The analytic sample comprised 910 children (461 were girls [50.7%]; 518 were African American [56.9%], and 392 were white [43.1%]) with genetic data from the Family Life Project, a longitudinal birth cohort of families living in predominantly low-income and rural communities in the United States (
18). (For a detailed description of the sample, sampling procedure, and data collection, see the
online supplement and reference
18.)
Measures
Exposure to intimate partner violence (IPV).
The child’s primary caregiver (in almost all instances [95%] the child’s biological mother) reported on IPV using the Conflict Tactics Scale (
19) at child ages 7, 15, and 24 months. An aggregate of the respondent’s own and the respondent’s partner’s physical and psychological aggression was used to create a quantitative index of violence exposure (range, 0–4) during the child’s first 2 years.
Fear-elicited emotional reactivity.
At 7, 15, and 24 months, children underwent a mask presentation procedure to induce fear (
20,
21). The experimenter wore four different masks, one at a time, while moving slowly from side to side in front of the child, saying the child’s name using a neutral tone of voice. At 7 months, children also underwent a barrier procedure and arm restraint procedure, and at 15 and 24 months, children underwent a toy removal procedure to induce frustration. We focus on the mask procedure given that it has been associated with the fear response and activity in the corticolimbic neural circuitry (
22) that are central to our theoretical model. (See the
online supplement for details on coding psychometric properties and Figure S2 in the
online supplement for cortisol levels prior to and following the stress paradigm at each time point, broken down by CATT haplotype and IPV exposure.)
Prolonged stress-induced cortisol reactivity.
Stress-induced changes in cortisol were measured at 7, 15, and 24 months. To assess stress-induced changes in cortisol, saliva samples were collected before the mask presentation and at 20 and 40 minutes after peak arousal to the procedure and later assayed for cortisol. (For further details, see the online supplement.)
Intellectual ability.
The Mental Development Index of the Bayley Scales of Infant Development (
23), administered at 15 months, and the full-scale estimate of IQ from the Wechsler Preschool and Primary Scale of Intelligence (
24), administered at 36 months, were used to assess the child’s cognitive abilities.
Maternal depressive symptoms.
Self-reported maternal depressive symptoms at child age 24 months were assessed with the Center for Epidemiological Studies Depression Scale (
25).
Executive function.
At 36, 48, and 60 months, children underwent an executive function assessment battery that included three inhibitory control tasks (spatial conflict arrows, silly sounds Stroop, animal go/no-go), two working memory tasks (working memory span, pick the picture), and one attention-shifting task (“something’s the same”) (
26). (For a detailed description of the tasks and the administration procedures, see the
online supplement and reference
26.)
Emotional and behavioral problems.
The child’s lead teacher at school in grades 1, 2, and 5 completed the Strengths and Difficulties Questionnaire (SDQ) (
27) to assess conduct problems, hyperactivity, emotional problems, and peer problems. The sum of the subscale scores was used to measure emotional and behavioral problems over time.
Reading and math ability.
In grades 1, 2, and 5, the letter-word identification and applied problems subtests of the Woodcock-Johnson III Tests of Achievement (
28) were administered to assess reading and math ability. W scores, appropriate for longitudinal analysis of change, were used in all analyses.
Genotypes.
DNA was extracted from both the child and the biological mother, and genotyping of rs9296158, rs3800373, rs1360780, and rs9470080 was conducted with the appropriate probes for a Taqman SNP Genotyping Assay using an allelic discrimination assay protocol (Applied Biosystems, Foster City, Calif.). In addition, genetic ancestry markers were assessed. (See the online supplement for genotyping and quality control procedures and genetic ancestry marker analyses.) These four FKBP5 SNPs were combined to determine the copy numbers of the rarer CATT haplotype carried by each individual, using PLINK (children: 432 noncarriers, 384 carriers of one CATT haplotype allele, and 94 carriers of two CATT haplotype alleles; mothers: 414 noncarriers, 365 carriers of one CATT haplotype allele, and 86 carriers of two CATT haplotype alleles). Haploview was used to determine the linkage disequilibrium structure of the FKBP5 SNPs (see Figure S3 in the online supplement).
Covariates.
Covariates included child sex, child state of residence, cumulative risk, household chaos, and genetic ancestry markers. Cumulative risk is a continuous score based on family income-to-needs ratio, constancy of spouse/partner living in the home, maternal education, hours of employment, household density, occupational prestige, and neighborhood noise/safety, measured at child age 7, 15, and 24 months (
18). Household chaos is rated as a continuous score based on the number of times the child moved to a new residence, changes in primary and secondary caregivers, and changes in the people within the household, at 7, 15, and 24 months. These scores are based on previous work with the Family Life Project (
18). To control for population stratification, the first six principal components derived from the ancestry-specific genotypes (see the
online supplement) were included in all analyses.
Statistical Analysis
Genotype differences in demographic and clinical characteristics were examined with chi-square and regression models. To examine prolonged stress-induced cortisol reactivity, we regressed cortisol levels from the earlier time points (cortisol levels at baseline and 20 minutes after the stress paradigm) and the covariates onto the cortisol levels measured 40 minutes after the stressor at each assessment (at 7, 15, and 24 months). The residuals from these models were used in our longitudinal cluster analyses. Multiple imputation was used to handle missing data (see the online supplement for imputation procedures and Table S1 in the online supplement for available data by outcome).
Analyses followed an additive genetic model in which individuals with increasing numbers of risk haplotypes (noncarrier=0, one CATT haplotype allele=1, and two CATT haplotype alleles=2) were compared and exposure to IPV was examined as a continuous measure. K-means-based longitudinal clustering was conducted to identify groups of participants with similar phenotype presentation across time. Two separate cluster analyses were conducted to account for the developmental stage at the time of measurement. The first cluster analysis included prolonged stress-induced cortisol reactivity and fear-elicited emotional reactivity measured at 7, 15, and 24 months. The second cluster analysis included reading and math ability and teacher-reported emotional and behavioral difficulties in grades 1, 2, and 5. The best clustering solution was determined using the Caliński and Harabasz criterion (CH index) (
29). To assess the developmental progression of children from the early development clusters to the school-age clusters, Cohen’s kappa test (
30) was conducted to infer the correspondence between two clustering results (see the
online supplement for details on the cluster analyses procedures).
The outcomes of the early development and school-age cluster analyses were ranked by phenotype severity. Ordinal logistic regression (
31) with a proportional odds regression was used to examine the main effect model (G or E), the additive model (G+E), and the multiplicative interactive model (G×E) of the CATT haplotype and IPV exposure on the ranked phenotypes. To determine model fit, likelihood ratio tests were used to compare each model to the reduced model. To complement these analyses, mixed linear growth models were conducted in R to illustrate the findings of the longitudinal cluster analyses for each outcome (see the
online supplement).
We carried out several sensitivity analyses to exclude alternative explanations and determine the specificity of the effects (see the online supplement and Figure S4.)
Results
No significant differences were observed in demographic or clinical characteristics between child genotypes (see Table S2 in the online supplement). For child outcomes, the correlation was lower among variables earlier in development compared with outcomes in early and middle childhood (see Figure S5 in the online supplement). However, the correlation between outcomes gradually increased across variables from 24 months onward, suggesting that convergences of developmental trajectories over the first 24 months are relevant for the prediction of later outcome.
Longitudinal Cluster Analyses
Longitudinal cluster analyses were used to identify individuals with similar prolonged stress-induced cortisol reactivity and emotion regulation over the first 2 years of life and behavioral and academic trajectories from grade 1 to grade 5. For both the early development and school-age cluster analyses, a two-cluster solution had the highest CH index (see Figure S6 in the online supplement). During early development, a cluster with low emotional reactivity and faster normalization of cortisol (cluster “low emotional reactivity/cortisol”; 51.1% of participants; see Figure S7 in the online supplement) separated from a cluster of children with higher emotional reactivity and prolonged normalization of cortisol (cluster “high emotional reactivity/cortisol”; 48.9% of participants). In the school-age children, a cluster with fewer emotional and behavioral problems and higher academic ability (cluster “low behavioral problems/high academic achievement”; 63.4% of participants) contrasted with a cluster with more behavioral problems and lower academic ability (cluster “high behavioral problems/low academic achievement”; 36.6% of participants). The clusters across the two developmental stages were in “slight agreement” (Cohen’s kappa=0.032, 95% CI=−0.034, 0.097, p=0.17).
The combined developmental clusters were ranked by severity of difficulties across time.
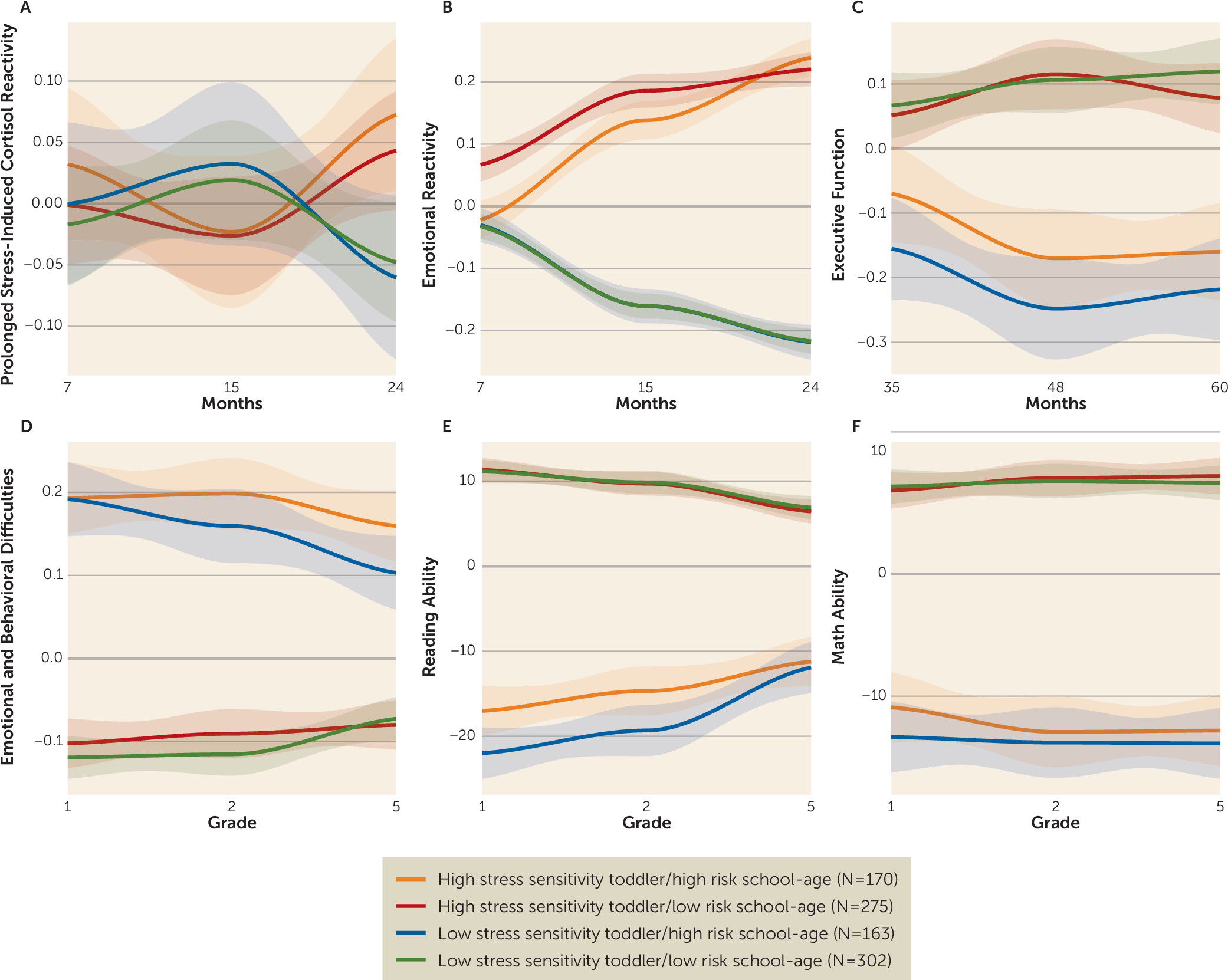
Figure 1 illustrates all the outcomes according to the four cluster groups. Children within the clusters of high emotional reactivity/cortisol in toddlerhood and high behavioral problems/low academic achievement later on had the most difficulties across time (high stress sensitivity toddler/high risk school-age, N=170). They were followed by children who presented with high levels of difficulties in the school-age analyses but not increased emotional reactivity during early development (low stress sensitivity toddler/high risk school-age, N=163). A third group of children presented with increased emotional reactivity difficulties during early childhood but no difficulties in middle childhood (high stress sensitivity toddler/low risk school-age, N=275). Children in the last group presented with both low emotional reactivity during early childhood and low emotion and behavioral difficulties and high reading ability in middle childhood (low stress sensitivity toddler/low risk school-age, N=302).
Ordinal Logistic Regression Analysis
Ordinal logistic regression revealed that executive function over the course of development, which was not used to define the cluster groups and was measured at time points between the early and school-age trajectories (at 36, 48, and 60 months), was significantly associated with the cluster groups (β=0.246, SE=0.034, p<0.001) (
Figure 1C). Children with elevated levels of prolonged stress-induced cortisol and emotional reactivity in toddlerhood also had the lowest executive function at all three time points.
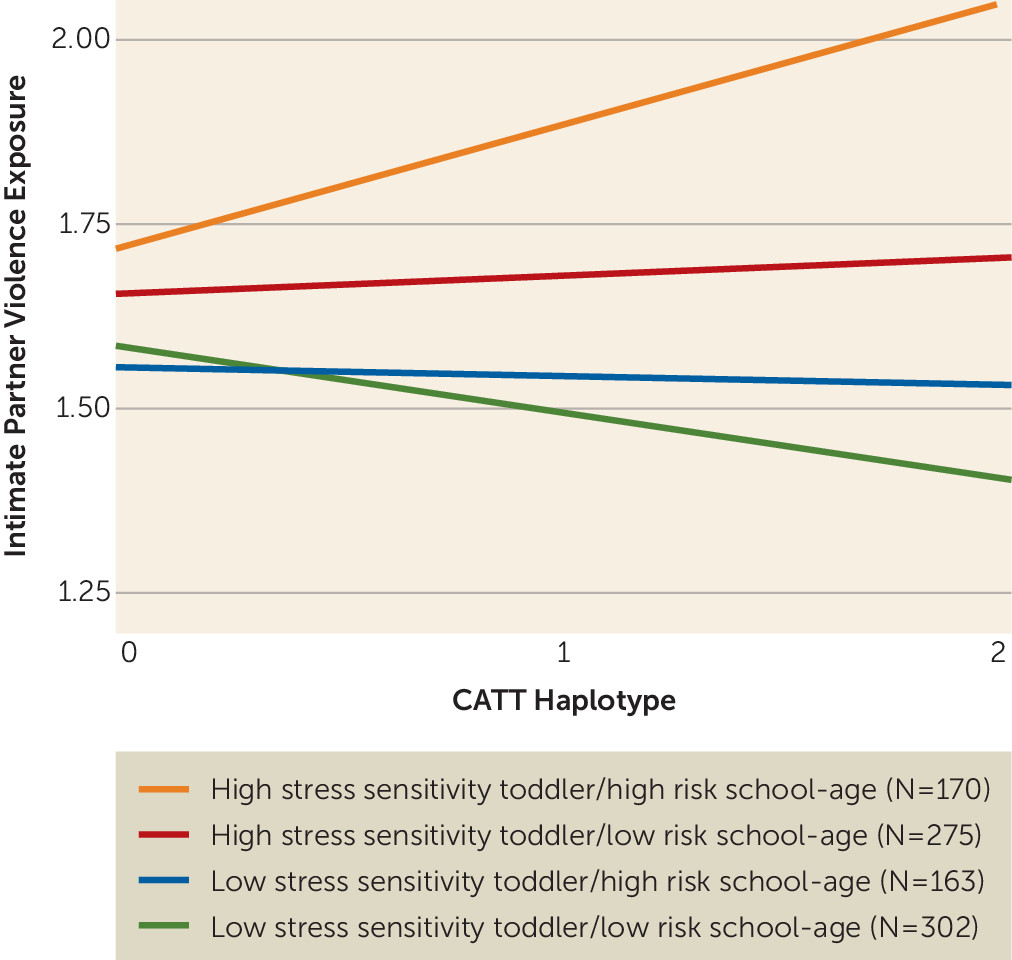
Next, we examined how the IPV exposure and
FKBP5 haplotype interaction was associated with the developmental trajectory a child belonged to. A model including the interaction of CATT haplotype by IPV exposure showed a stronger association with the cluster groups than both the null model (p=0.005) and the additive model (p=0.037; see
Table 1 for model comparisons), providing evidence that the CATT haplotype-by-IPV interaction is associated with the clustering orders (
Figure 2). Children belonging to the high stress sensitivity toddler/high risk school-age cluster had the highest IPV exposure and the highest proportion of carriers of two copies of the CATT risk haplotype. Specifically, 29.7% of participants carrying two copies of the CATT haplotype and with above-average levels of IPV exposure (mean IPV exposure for the sample, >0.847) belonged to the high stress sensitivity toddler/high risk school-age cluster, compared with 14.2% of participants carrying two copies of the CATT haplotype with lower levels of IPV exposure. The odds ratio of carrying two copies of the CATT haplotype and having high IPV exposure and being in the group of children with an at-risk developmental trajectory (high stress sensitivity toddler/high risk school-age cluster) was 4.15 (p=0.031, 95% CI=0.99, 21.12) in comparison to being in the group of children with a low-risk developmental trajectory (low stress sensitivity toddler/low risk school-age cluster).
Mixed Linear Models
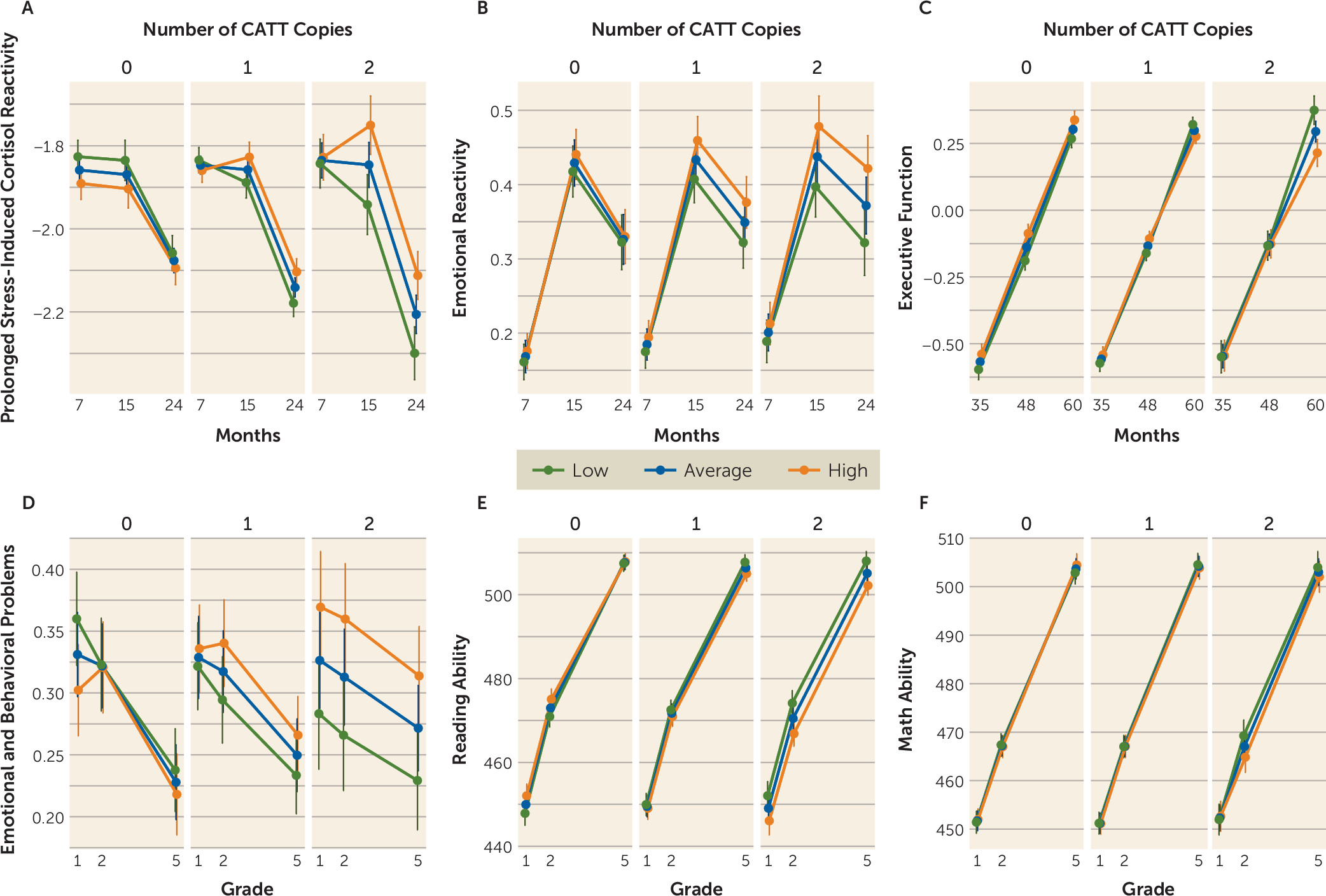
Mixed-model analyses were used to more explicitly visualize the findings from the longitudinal cluster analyses. The CATT haplotype-by-IPV interaction significantly predicted prolonged stress-induced cortisol and emotional reactivity at 15 and 24 months (p=0.035 and p=0.038, respectively), executive function at 60 months (p=0.029), and emotional and behavioral problems (p=0.020) and reading ability (p=0.035) in school grades 1, 2, and 5 (Cohen’s d values ranging from 0.13 for prolonged stress-induced cortisol at 15 months to 0.77 for emotional and behavioral problems in grade 5, for the significant findings). These analyses are illustrated in
Figure 3 and detailed further in the
online supplement.
Sensitivity Analyses
The IPV and CATT haplotype interaction remained a significant predictor of reading and math ability after adjusting for general intelligence. The interaction also remained a significant predictor of cluster group assignment when controlling for maternal depressive symptoms. Maternal CATT haplotype, independently or in combination with IPV, was not associated with cluster group assignment. The interaction between cumulative risk and CATT haplotype did not significantly predict any of the self-regulation or school-age outcomes (p values >0.050). The three-way interaction between IPV, CATT haplotype, and ethnicity did not significantly predict cluster groups (p=0.250). Lastly, the CATT haplotype-by-IPV interaction did not predict any measure of general intelligence, providing support for the interaction effect being specific to self-regulation outcomes.
Discussion
As predicted by the model of self-regulation, exposure to high levels of IPV before age 2 among FKBP5 risk haplotype carriers was associated with higher levels of physiological and emotional reactivity, with consequences for emotional and cognitive development through middle childhood.
The findings are consistent with the mounting research on the dynamic relationship between the stress hormone system and self-regulation and how their interaction affects an individual’s functioning over time (
32). The associations were confined to self-regulation outcomes and were not observed for intellectual ability. Previous molecular and endocrine findings show that an enhanced up-regulation of
FKBP5 during the acute stress phase (which has been associated with the CATT haplotype) leads to impaired negative feedback of the stress hormone system and prolonged cortisol responses by reducing glucocorticoid receptor sensitivity and thus negative feedback mechanisms (see reference
5 for a review). Therefore, an altered endocrine response to stress in the presence of a chronic stressor, such as IPV, would be expected to influence behavioral outcomes. In accordance, the collective findings of this study indicate that children carrying the CATT haplotype who are exposed to IPV during the first 2 years of life are significantly more likely to display early prolonged cortisol responses in parallel with differences in emotion regulation, followed by later cognitive and behavioral differences. This may suggest that early variation in emotion and stress hormone response regulation determined by environmental and genetic risk may drive aspects of behavioral adaptation over the course of development.
The observed behavioral differences in this study may be the result of increased FKBP5 expression in the developing brain. In experimental animals, higher FKBP5 expression in specific brain regions has been associated with increased anxiety-related behavior as well as reduced stress coping (see reference
33 for a review). Human neuroimaging studies have shown an association between the high
FKBP5 expression CATT haplotype and changes in the volume and connectivity of the hippocampus, frontal cortex, and amygdala (
34). Furthermore, a recent postmortem study (
33) revealed decreased synaptic spine density in the medial orbitofrontal cortex associated with increased FKBP5 expression (which would be predicted in CATT carriers with early-life stress exposure [
35]), suggesting that high FKBP5 expression may be associated with the biological systems necessary for learning and memory. In fact, increases in FKBP5 not only would affect glucocorticoid receptor regulation but also would alter a number of downstream pathways that FKBP5 influences via protein/protein interaction (
36). These include systems associated with neuronal function and synaptic plasticity and could thus have a direct impact on these brain circuits. Studies are needed to examine the effects of
FKBP5-by-early-life stress, such as IPV exposure, on emotional circuit activation throughout development.
This study adds to the growing literature indicating that
FKBP5 and early-life stress interact in shaping outcomes across multiple domains of functioning (
5). In this study, the clusters at each developmental stage were in “slight agreement” according to the Cohen’s kappa value. This relatively low correlation between child outcomes and the clusters likely reflects processes of equifinality (multiple pathways ending with the same outcome) and multifinality (children with similar risks ending with different outcomes) (
37,
38), as is commonly presented in the literature (
37). Moreover, our analyses were confined to exposure to IPV before age 2 and we did not adjust for environmental influences after that period. Notably, despite not having controlled for environmental influences past age 2, we still found that children characterized by the combination of the
FKBP5 risk haplotype and IPV exposure before age 2 were overrepresented in the high-risk outcome trajectories across all age periods.
This study has a number of strengths (e.g., a multi-informant approach, biological measures, and longitudinal assessment), but also several limitations. First, participants with missing IPV data were more likely to be African American and to be characterized by higher cumulative risk. In an effort to overcome this limitation, we employed state-of-the-art procedures to address missing data (see the
online supplement). Second, the diverse ethnic background of the cohort may confound the G×E analysis. To avoid false positive results confounded by population stratification, we used ancestry-informative genetic markers as covariates in all analyses and tested for the three-way interaction of IPV with
FKBP5 and race/ethnicity. We found no evidence that ethnicity confounds the observed G×E interaction. Similarly, our sample was composed predominantly of children of low socioeconomic status. Poverty is a well-studied early-life stress associated with poor physical and mental health. Notably, our measure of cumulative risk, which incorporated socioeconomic status, did not significantly predict prolonged stress-induced cortisol reactivity and emotional reactivity in this study, whereas exposure to IPV did. Hence, in order to investigate how factors that influence prolonged stress-induced cortisol reactivity and emotional reactivity in early childhood affect later outcomes, we believe that IPV is the best suited exposure in this cohort. Third, it was beyond the scope of the study to disentangle whether the increased risk for emotional and behavioral symptoms and poor academic achievement observed were due to other stressors, such as prenatal stress due to IPV exposure during gestation. The association of other types of early adversity with HPA reactivity and longitudinal child outcomes remain an important future research venture. Fourth, this study focused on the influence of the G×E interaction on self-regulation from infancy to middle childhood. A recent cross-sectional study suggested that
FKBP5 may interact with early-life stress to predict self-regulation outcomes, namely, rumination and catastrophizing, during adolescence (
39). Further longitudinal data are required to determine whether this interaction influences adult self-regulation outcomes.
In conclusion, and consistent with the hypothesized model of self-regulation, functional FKBP5 haplotypes interacted with IPV exposure, resulting in long-term and likely interconnected effects on endocrine, emotional, behavioral, and cognitive functioning throughout development into middle childhood. These findings provide valuable insight into potential early shared mechanisms underlying the diverse mental health and cognitive outcomes associated with early adversity-by-FKBP5 interactions and could have important implications for identifying at-risk children and intervention targets.
Acknowledgments
The authors thank the many families and research assistants who made this study possible.
Acknowledgments
The Family Life Project key investigators include Lynne Vernon-Feagans, University of North Carolina at Chapel Hill; Mark T. Greenberg, Pennsylvania State University; Clancy B. Blair, New York University; Margaret R. Burchinal, University of North Carolina at Chapel Hill; Martha Cox, University of North Carolina at Chapel Hill; Patricia T. Garrett-Peters, University of North Carolina at Chapel Hill; Jennifer L. Frank, Pennsylvania State University; W. Roger Mills-Koonce, University of North Carolina–Greensboro; and Michael T. Willoughby, RTI International.