Delusions occur across a range of psychotic disorders and are characterized by distressing false beliefs that preoccupy, are held with conviction, and disrupt lives. Delusions have a complex phenomenology, and theories of causative and maintenance factors are possibly just as complex (
1–
5). Recent conceptualizations of the cognitive architecture of delusions have modeled how aberrant beliefs are overlearned and become immutable (
1,
6–
8). It has been argued that the preoccupation and conviction with which beliefs are held may stem from cognitive biases against incorporating into one’s beliefs evidence to the contrary (
2,
4). These erroneous beliefs are potentially further augmented through exaggerated subcortical dopaminergic reinforcement (
9,
10). Consistent with this formulation, related hallucinatory phenomena in at-risk mental states were recently observed to result from biasing prior expectations over incoming (contrary) sensory evidence (
11). At the level of brain function, these computational biases appear to result from deficits in feedforward information updating, which have also been found in subclinical psychotic experiences (
11). More specific to the abstract information processing in delusional beliefs, we previously found that deficits in new information updating in favor of prior beliefs was also associated with reduced feedforward effective connectivity across posterior to prefrontal cortices (
1). These changes were unlikely to be epiphenomena of treatment, because analogous information processing changes also were observed in unaffected siblings of patients with schizophrenia (
1).
Our goal in this study was to extend recent observations linking delusional psychopathology, genetic risk for schizophrenia, and deficits in feedforward parietal-to-prefrontal connectivity during updating of new context information (
1). Recent models of delusional and perceptual psychopathology suggest deficits in feedforward cortical integration of new information into belief models, leading to dysfunctional strong prior assumptions and consequent belief and perceptual disturbances (
1,
12,
13). We hypothesized that these effects would also be reflected in updating and manipulating context information in working memory. Cortical integration of new information into beliefs occurs along a hierarchy of overlapping processes, including executive aspects of working memory, Bayesian learning and reasoning, and episodic memory (
14,
15). We reasoned that because executive aspects of working memory form the base of these hierarchical processes and involve updating prior information with new content, they should be critically engaged in the updating of new information into belief models that are putatively dysfunctional in delusions, and should also be associated with the underlying genetic mechanisms of schizophrenia (
1,
12,
13). This formulation is supported by the observed relationships between tasks that involve updating new information in contextual working memory and the Bayesian integration of this new information in working memory, upon which hierarchically more complex belief processing subsequently occurs (
1,
14,
15). Recent work has also underscored relationships between deficits in updating or manipulating information in contextual working memory, feedforward parietal-prefrontal connectivity, and delusions (
1,
13,
16,
17). We therefore predicted that if feedforward parietal-prefrontal connectivity while manipulating prior information held in working memory might relate to delusional psychopathology in patients with schizophrenia, these neural functions might also relate to genetic mechanisms of illness, rather than confounders associated with medication or chronic ill health. If so, we would expect that there would be relationships between feedforward parietal-prefrontal connectivity and polygenic risk for disease, particularly in healthy individuals.
We also aimed in this study to examine a substantial issue related to interindividual variability in cortical function and connectivity, which is particularly pronounced in the prefrontal cortical processing we examined here (
18–
20). Because each patient’s optimal neural function for a given cognitive task likely varies, a given patient’s deviation from his or her theoretical optimum should closely relate to cognitive dysfunction and psychopathology, if indeed the specific neural function is implicated in these disease mechanisms. To examine this, we leveraged data in healthy control samples who performed analogous context updating tasks. We created support vector regression models of how target feedforward parietal-prefrontal network connectivity during context manipulation in working memory may be predicted by connectivity during simple maintenance of information, the latter a process found to be less dysfunctional in schizophrenia than is context updating (
21–
23). The machine learning models thus derived in healthy subjects were then applied to each patient to determine what his or her target “normal” quantitative feedforward connectivity should be. This novel strategy takes inspiration from related machine learning decoder approaches that utilize surrogate subjects to predict activation patterns for an individual subject (
24).
Methods
Participants
We examined 143 healthy control subjects and 66 patients with schizophrenia as they performed an event-related working memory task during MRI scanning. Participants, who were enrolled as part of the Clinical Brain Disorders Branch Sibling Study (
25), were between 18 and 45 years of age (
Table 1) and were right-handed. They were interviewed by a research psychiatrist using the Structured Clinical Interview for DSM-IV Axis I Disorders, and completed a neurological examination and a battery of neuropsychological tests. Exclusion criteria included an IQ <70, a history of prolonged substance abuse or significant medical problems, and any abnormalities found by EEG or MRI. All participants gave written consent before participation. The study was approved by the National Institute of Mental Health Institutional Review Board. The individuals in this sample were not part of our earlier study (
1).
Cognitive Task Paradigm
Blood-oxygen-level-dependent (BOLD) functional MRI data were acquired for each participant after a brief training period (∼10 minutes). The event-related working memory paradigm has been described in detail elsewhere (
26). In each trial, participants first encoded two integer numbers presented over 1 second and retained this context information in working memory across a jittered interval of 3–5 seconds. In maintenance trials, participants subsequently responded to cues as to which of the two numbers was “larger” or “smaller” within 2 seconds. In the manipulation trials, participants had to perform a mental subtraction of 2 or 3 on one of the two context numbers before the “larger” or “smaller” evaluation within 2 seconds. There were 28 trials of working memory manipulation and 28 trials of working memory maintenance, counterbalanced for trial type and numerical size order, over about 9 minutes.
Imaging Parameters and Analyses
T2*-weighted echo planar imaging (EPI) images with BOLD contrast were obtained with a 3-T MRI scanner (GE, Milwaukee) using a standard GE head coil (64×64×24 matrix with 3.75×3.75×6.0 mm spatial resolution, repetition time=2000 ms, echo time=28 ms, flip angle=90°, field of view=24×24 cm) while participants performed the cognitive task. The first four scans were discarded to allow for signal saturation.
The functional imaging data were preprocessed and analyzed using the general linear model for event-related designs in SPM12 (Wellcome Trust Centre for Neuroimaging, London). The functional images were corrected for differences in acquisition time between slices for each whole-brain volume and realigned to correct for head movement. Six movement parameters (translation: x, y, z; and rotation: roll, pitch, yaw) were included in the statistical model as covariates of no interest. The functional images were normalized to a standard EPI Montreal Neurological Institute template and then spatially smoothed using an isotropic Gaussian kernel of 8 mm full width half maximum.
A random-effects event-related statistical analysis was performed at two levels. In the first level, the onsets and durations of each trial for each task condition were convolved with a canonical hemodynamic response function and modeled using a general linear model on an individual subject basis. The realignment parameters were included as additional regressors of no interest. Data were high-pass filtered at 1/128 Hz. Random-effects analyses at the second (group) level were then conducted based on statistical parameter maps from each individual participant to allow population-level inference. Group-level activation maps were thresholded at a voxel-level whole brain p threshold of <0.05 with family-wise error correction for multiple comparisons unless otherwise stated.
Dynamic Causal Modeling
We used dynamic causal modeling (DCM) (
27) as implemented in SPM12 (DCM10) to examine how parietal and prefrontal brain regions interacted. We focused on the left parietal (coordinates, −38, −58, 38) and prefrontal (−44, 30, 16) regions implicated previously in context updating dysfunction in delusions (
1), and we examined the corresponding parcels defined by the Human Connectome Project (
28) in the parietal (the dorsal lateral intraparietal area [LIPd]) and prefrontal (Brodmann’s area 46) cortex encompassing these peaks. Time series during working memory context updating (manipulation) were extracted from each of the two regions of interest for each individual, masked by the conjunction of the respective cortical parcel, the group-level activation mask at p<0.05, voxelwise whole brain family-wise error corrected for multiple comparisons, and an individual subject-level activation at p<0.05. Deterministic bilinear DCM models comprising all seven possible input and pairing combinations of these two regions of interest (
29) were then specified (
Figure 1B shows this at the dorsolateral prefrontal cortex and parietal cortex, although this also applies to all region-of-interest pairs in the machine learning models below). The strength and direction of regional interactions were then estimated, elucidating how regional neural activity and their interactions were influenced by cognitive inputs during working memory context updating, as well as how these neuronal effects were biophysically linked to form BOLD signals (
27). Bayesian model averaging was used to generate weighted task-related connectivity averages in each direction, for each pair of nodes, based on the posterior model probability (
30). Using these results, we then examined task-related modulation of regional connectivity across participants and relationships with psychopathology and polygenic risk for schizophrenia (
31).
Polygenic Risk for Schizophrenia
Polygenic risk scores for schizophrenia were calculated for each control subject as a measure of genomic risk for schizophrenia, based on the most recent genome-wide association study (GWAS) (
31). We obtained odds ratios of 102,217 index single-nucleotide polymorphisms (SNPs) from a meta-analysis of Psychiatric Genomics Consortium phase 2 (PGC2) GWAS using data sets excluding the discovery sample (PGC 2014, non-Lieber sample PGC2 GWAS). These SNPs were linkage disequilibrium independent (R
2<0.1). We then calculated a weighted sum of risk alleles for schizophrenia by summing the imputation probability for the reference allele of the index SNP, weighted by the natural log of the odds ratio of association with schizophrenia, at each independent locus across the whole genome (
31,
32). In this analysis, we used the polygenic risk score calculated using the conservative PGC2 GWAS association p value of 5×10
−8.
Defining Individual Patients’ Optimal Parietal-Prefrontal Connectivity
We defined and tested the validity of each individual patient’s putative optimal (normal) parietal-prefrontal connectivity at working memory context updating. We first created a linear support vector regression (SVR) model, in healthy control subjects, of how feedforward parietal-prefrontal network connectivity during context updating may be predicted by connectivities from prefrontal and parietal regions during simple working memory maintenance, a process that is less dysfunctional in schizophrenia than context updating (
21–
23). We included 31 parcels in the frontal and parietal cortex that were robustly engaged during working memory maintenance in the patient and control samples (p<0.05, whole-brain voxel-wise family-wise error corrected in each separate patient or control sample). Time series during working memory maintenance were then extracted from the region of interest for each individual, masked by the conjunction of the respective cortical parcel, group-level activation mask at p<0.05, whole brain voxelwise family-wise error corrected for multiple comparisons, and the individual subject-level activation at p<0.05. Deterministic DCM models comprising all possible pairings across these 31 regions of interest (
29) were specified, giving 465 pairs of regions. From each pair of regions of interest, we determined the Bayesian model averaging over all seven possible models within which a pair of regions could effectively interact (
Figure 1B, and in reference
29). This resulted in a directed matrix of effective connectivity during working memory maintenance, predicting in SVR modeling in healthy control subjects the target parietal-prefrontal feedforward connectivity during working memory context updating. SVR was conducted in MATLAB using the implementation “fitrsvm” with a linear kernel, automatic hyperparameter tuning, and sequential minimal optimization. We examined the in-sample predictive validity using a leave-one-out correlation analysis of the left-out predicted parietal-prefrontal connectivity compared with the actual connectivity. We also examined predictions in more conservative approaches, including fivefold cross-validation in SVR with a held-out 20% test sample, and elastic net regression (
33) with alpha at 0.5, also with a held-out 20% test sample.
More importantly, we subsequently tested the validity of this modeling approach in the patients. The SVR models thus derived in healthy control subjects were applied to each patient to determine what that individual patient’s target “normal” feedforward connectivity should be at context updating during working memory manipulation, given the patient’s connectivity pattern during the relatively less dysfunctional working memory maintenance. We examined each patient’s predicted normal connectivity and the deviation from his or her actual connectivity, as well as the extent to which this deviation correlated with delusional psychopathology, hypothesized previously to be associated with the feedforward connectivity deficits. We then tested the extent to which the connectivity-derived support vector predictions may outperform correlations with delusions derived by simply comparing between patient feedforward connectivity and mean control feedforward connectivity, and also by feedforward connectivity deviations across patients and control subjects uninformed by machine learning, the latter generated by random pairings between patients and control subjects over 10,000 permutations.
Results
Demographic Characteristics and Task Performance
Across healthy subjects (N=143) and schizophrenia patients (N=66), age and parental education were similar (
Table 1). The mean duration of illness among patients was 8.2 years (SD=7.82) and the mean age at onset was 19.5 years (SD=4.2). The patients’ mean total positive and negative symptom score was 54 (SD=24), and their mean dosage of antipsychotic medication was 298 mg/day (SD=389) of chlorpromazine equivalents.
Working memory manipulation was more difficult (less accurate) than working memory maintenance, and patients generally performed more poorly in working memory accuracy and reaction time (
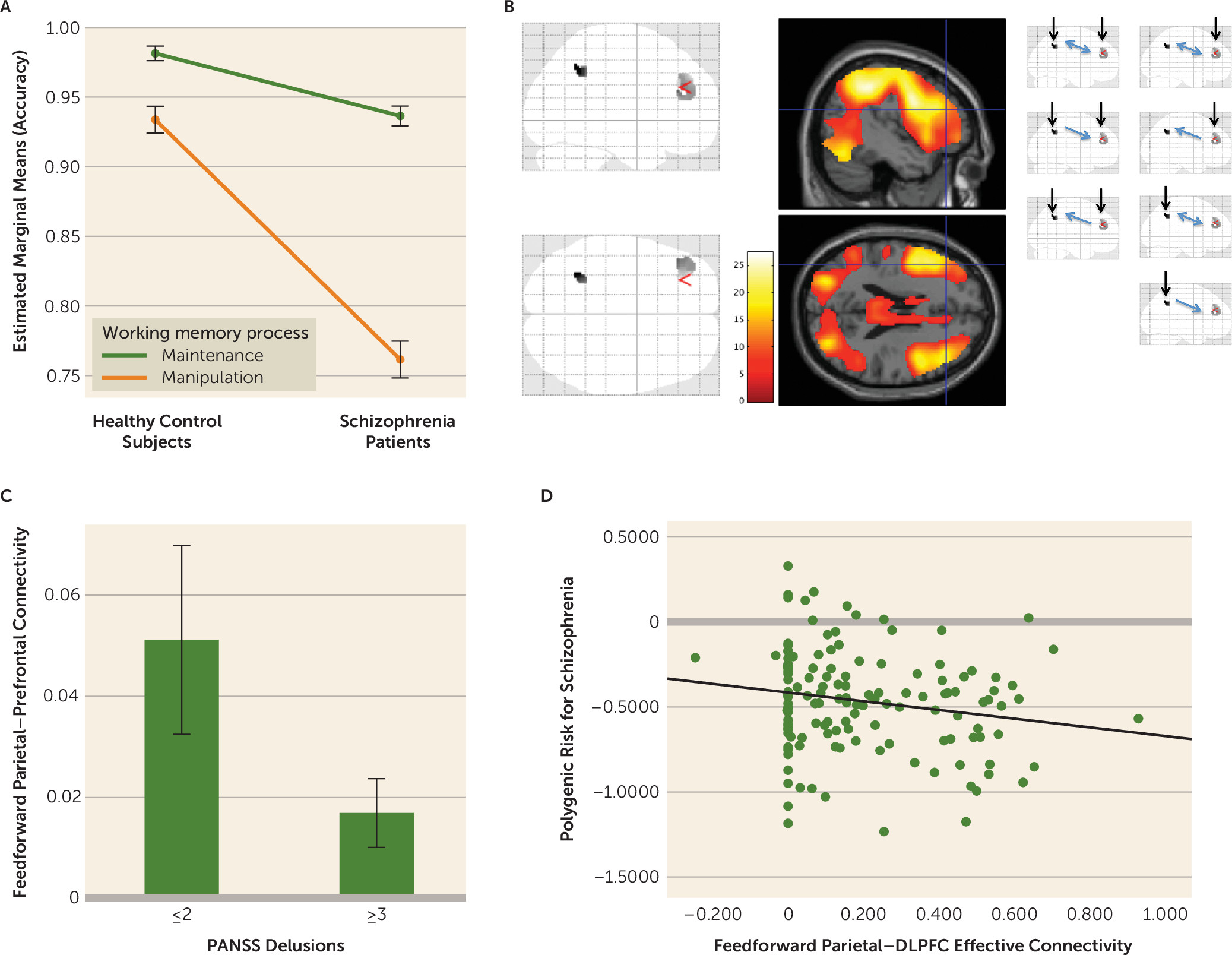
Table 1). However, working memory manipulation was disproportionately more dysfunctional than working memory maintenance in patients (working memory process-by-diagnosis interaction, F=63.9, df=1, 217, p<0.001) (
Figure 1A).
Parietal-Prefrontal Feedforward Connectivity
In this report, we focus on the left LIPd and area 46 regions of interest (Human Connectome Project parcellation notations [
28]), which correspond to regions where we previously found parietal and prefrontal effects associated with delusions and context updating in schizophrenia (
1). Peaks in the left LIPd and area 46 regions of interest were significantly engaged during context updating in working memory manipulation at the group level in control subjects and in patients (p<0.05, voxel-wise whole-brain family-wise error corrected for multiple comparisons, in each control and patient group) (
Figure 1B; see also Table S1 in the
online supplement). Effective connectivity across these two regions of interest during context updating in working memory manipulation were engaged at feedforward (parietal to prefrontal) and feedback (prefrontal to parietal) in control subjects (p<0.001; see Table S2 in the
online supplement) and in schizophrenia patients (p<0.001; see Table S4 in the
online supplement). Connectivity in both directions was relatively reduced in patients between the LIPd and area 46 regions of interest (p<0.001).
Within patients with schizophrenia (N=66), we examined the hypothesized role of reduced parietal-prefrontal feedforward effective connectivity in the left LIPd and area 46 regions at context updating during working memory manipulation in delusional psychopathology, as suggested previously (
1). Individuals with a score ≥3 for delusions on the Positive and Negative Syndrome Scale tended to have relatively reduced parietal-prefrontal feedforward effective connectivity (t=1.97, p=0.05) (
Figure 1C). Effects in the opposite feedback direction were not significant.
We then tested whether these feedforward parietal-to-prefrontal neural functions were related to putative genetic mechanisms of illness, rather than to confounders associated with medications or chronic ill health, through effects on polygenic risk for schizophrenia in healthy individuals. Left LIPd to area 46 feedforward effective connectivity during working memory manipulation was reduced in association with increased polygenic risk for schizophrenia (r=−0.19, p<0.02) (
Figure 1D). Effects in the opposite feedback direction were not significant.
Defining Individual Patients’ Optimal Parietal-Prefrontal Connectivity
If indeed dysfunction in parietal-prefrontal feedforward effective connectivity during updating of new information into context is related to delusions, the degree to which an individual patient’s connectivity deviates from his or her “optimum” value should also be related to dysfunction with delusions, particularly if the connectivity optimum reflects a relevant target to normalize psychopathology. To define an individual subject’s parietal-prefrontal connectivity optimum during working memory manipulation, we first examined 31 of the most activated parcels in the prefrontal and parietal cortices during working memory maintenance in control subjects (peak activation in each parcel of p<0.05, voxel-wise whole-brain family-wise error corrected for multiple comparisons; see Table S3 in the
online supplement). From these brain parcels, we computed the effective connectivity from each pair of nodes in both directions, giving 930 pairwise effective connectivity values during working memory maintenance. As noted previously, working memory maintenance performance accuracy was relatively less dysfunctional in patients (Cohen’s d=0.67 for maintenance and d=2.11 for manipulation, relative to control subjects) (
Figure 1A). However, in healthy control subjects (N=143), within the working memory maintenance prefrontal-parietal connectivity patterns in leave-one-out SVR models, feedforward parietal-prefrontal connectivity at LIPd to area 46 during working memory manipulation was robustly predicted (N=143, r>0.9, p<0.001; see Figure 1A in the
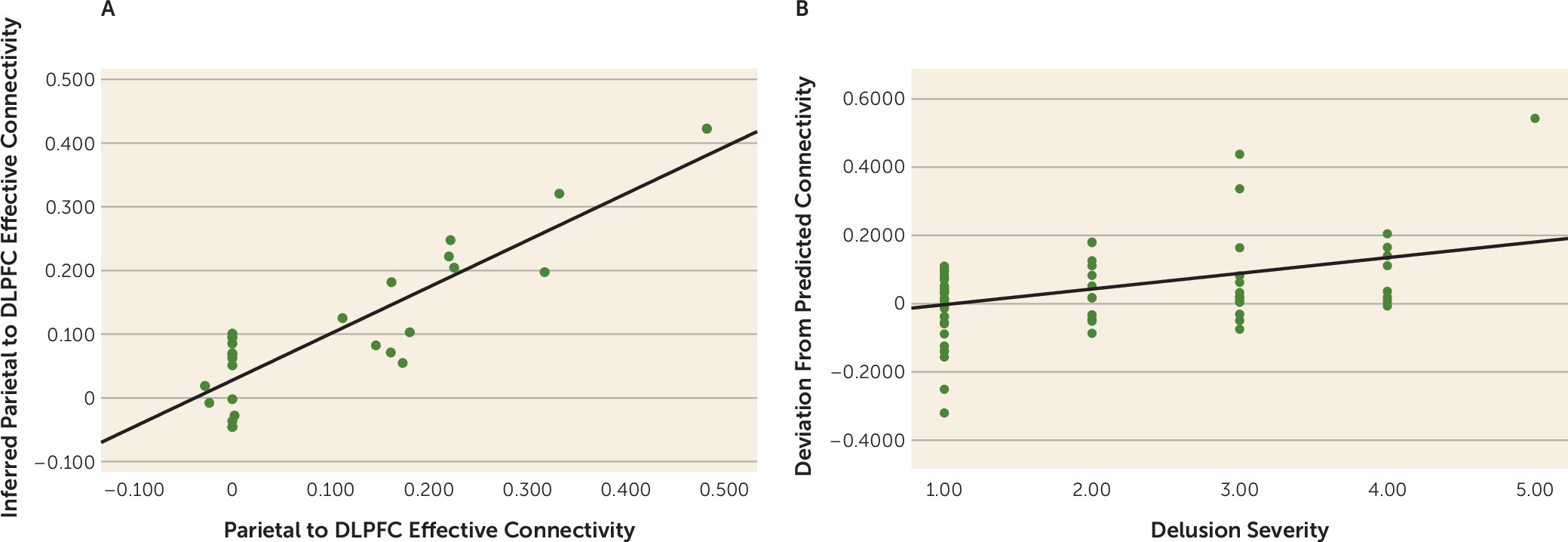
online supplement). More conservative SVR models trained with 80% of the healthy sample (N=116) and tested on a held-out 20% sample also gave robust predictions (N=27, r>0.80, p<0.001) (
Figure 2A). Elastic net regularization and variable selection (with alpha at 0.5), which effectively tackles model fitting where the number of variables exceeds that of subjects (
33), yielded connectivity predictions in the held-out sample with an effect size reasonably similar to that of SVR (N=27; r=0.60, p<0.001; see Figure 1B in the
online supplement).
We then reasoned that, given working memory maintenance connectivity from patients, we might predict their corresponding optimized working memory manipulation feedforward connectivity, using the SVR model independently derived from healthy control subjects. We tested the extent to which this individualized metric may be clinically significant, and found that patients’ deviations from this metric was associated with delusions (r=0.41, p<0.001) (
Figure 2B). On the other hand, deviation between each patient’s connectivity and mean control connectivity did not correlate with psychopathology (p>0.17). Deviation between each patient’s parietal-prefrontal connectivity and randomly paired control subjects’ parietal-prefrontal connectivity also did not correlate with psychopathology, and yielded an average r of 0.01 (p=0.52) over 10,000 iterations, with 2/10,000 instances of performance equal to or better than that of the support vector machine (r≥0.41). We further tested the specificity to delusional psychopathology and found no significant correlation between each patient’s deviation from modeled “optimal” feedforward connectivity with negative syndrome score (p>0.12) or with general symptom score (p>0.8). There was a smaller correlation with positive syndrome score (r=0.25, p=0.03), and, within the positive syndrome, correlation with hallucinations (r=0.26, p=0.02), as well as the larger effect with delusions noted above. In a regression of the connectivity deviation against hallucinations and delusions, the hallucination effect disappeared (p>0.8) and the delusion effect remained (beta=0.41, p=0.01), suggesting a degree of specificity for delusions.
Discussion
In healthy individuals, we observed that relative deficits in feedforward parietal-prefrontal connectivity during updating of new information into context during working memory manipulation is associated with higher polygenic risk for schizophrenia. In schizophrenia patients, we replicate, in an independent sample, earlier observations that this parietal-prefrontal effective connectivity relates to delusional psychopathology (
1,
17), although as expected, given the particular variability in prefrontal parietal function (
18–
20), these effects are not large (Cohen’s d<0.45). We then examined an estimate of optimal feedforward connectivity during working memory manipulation in patients, derived from the connectivity patterns during a less dysfunctional working memory maintenance task. Using a machine learning model derived from healthy subjects, larger deviations in feedforward parietal-prefrontal connectivity from predicted optima also related to delusional psychopathology in patients.
These observations are consistent with our previous findings in a nonoverlapping sample (
1), as well as that of others (
12,
17), on the role of feedforward information processing deficits in delusional psychopathology and genetic risk for schizophrenia. Individualized predictions of feedforward parietal-prefrontal connectivity during integration of new context information in working memory may thus serve as a potential biomarker to uncover underlying genetic mechanisms associated with information integration deficits in delusions.
Our results are consistent with a model of hierarchical posterior-to-prefrontal cortical networks (
14,
15) that mediates feedforward integration of new relevant context information (
1,
6,
7,
9,
34). Dysfunction of these circuits relates to the overestimated likelihoods of preexisting-belief models and their overreinforcement in delusions, which is suggested to be implicated also in the underlying genetic architecture of psychosis (
1). Prefrontal dysfunction and aberrant prefrontal-parietal connectivity have also long been implicated in schizophrenia and basic models of psychosis (
20,
35–
38).
A challenge in defining individual patient targets for potential therapeutics, however, is the substantial interindividual variability, particularly in prefrontal cortical functional (
18,
19) and effective connectivity (
39). This would affect how one might define appropriate targets to benchmark new treatment strategies based on brain function. A promising “hyperalignment” decoder approach recently utilized visual inferior temporal voxel patterns from surrogate subjects to predict voxel patterns for an individual with phobias without exposure to feared stimuli, where the predicted data outperformed that derived at the individual-subject level (
24). In terms of feedforward effective connectivity, we utilized an analogous strategy to infer target connectivity associated with context updating based on computationally inferring a patient’s connectivity pattern from models derived from the healthy participants’ data. We inferred from these data what the optimized individual patient-specific “normal” feedforward parietal-prefrontal connectivity targets would be. To the extent that patient-specific deviation from optima in this cortical feedforward connectivity may be clinically relevant, we found that these predictions do relate to psychopathology associated with these same connections.
The parietal-prefrontal feedforward connectivity target engaged by context task stimuli similar to that reported here (
1,
17), and prefrontal activation (
9), have been associated with the cognitive architecture of delusions as well as genetic risk for psychosis (
1). However, as it is likely that there are other neural connections that influence delusions beyond our regions of interest, a larger set of brain regions and larger samples should be explored. This should find some degree of feedback, and circular feedforward and feedback effects, conceptualized in the complex neural dynamics of delusions (
40). Our pairwise DCMs across multiple regions (
29) in the machine learning models is a pragmatic approach to the computational limitations in estimating large-scale task-modulated effective connectivity. However, this has drawbacks because each pair of brain interactions was considered independently of the others. Recent regression DCM approaches to large networks were focused on linear models that do not as yet include cognitive-task modulatory effects (
41) critical for our approach. However, future developments, such as task-modulated regression DCMs (
41) and augmented modeling of neural interactions (
42), could improve the biological and clinical validity of approaches such as ours. Nevertheless, we posit that feedforward parietal-prefrontal connectivity during information updating appears to be at least one target associated with the cognitive and genetic underpinnings of delusional psychopathology in schizophrenia, and perhaps in other psychotic conditions. The extent to which this circuit dysfunction might be causal would be important to determine, potentially with neurofeedback or other therapeutic strategies in functional MRI or EEG. Such new strategies may complement treatment of residual psychopathology, often refractory to pharmacology or cognitive-behavioral therapies.
Acknowledgments
The authors thank Dr. Hakwan Lau for valuable conversations.