Alcohol use is a leading cause of global disease burden, and alcohol use disorder (AUD) is a chronic, relapsing illness associated with significant medical morbidity and mortality worldwide (
1). Currently approved medications to treat AUD show modest therapeutic efficacy, partly because no specific alcohol–related pathophysiology has been identified as a clinical moderator of treatment response (
2,
3). Cessation of chronic, heavy alcohol use frequently increases the likelihood of alcohol withdrawal symptoms. These symptoms may be accompanied by increases in anxiety, depressive symptoms, and sleep difficulties, and together they encompass an increased alcohol abstinence–related distress state that is associated with high alcohol craving and increased risk of alcohol relapse and poor treatment outcomes (
4,
5). While treatment of alcohol withdrawal syndrome addresses acute alcohol-related delirium tremens, seizures, and mortality risk (
6), alcohol withdrawal and abstinence symptoms occur without alcohol withdrawal syndrome and are not systematically assessed as a prognostic marker of risk of postdetoxification relapse and treatment failure, and there are no specific treatments available to address such abstinence-related pathophysiology to improve drinking outcomes in AUD.
Neuroscience and human laboratory research to understand brain and clinical effects of chronic heavy alcohol intake, abstinence symptoms, and history of alcohol detoxifications indicate disrupted functioning of the medial prefrontal cortex, which is involved in regulating stress biology, reward, behavioral choices, and decisions (
4,
5,
7). Patients with AUD in early alcohol abstinence show parallel disruptions in peripheral stress physiology and in medial prefrontal cortex circuit functioning, which in turn are associated with higher stress and cue reactivity and increased risk of alcohol relapse and treatment failure (
4,
8,
9). This growing body of evidence suggests that abstinence-related disruption of stress biology in AUD may need specific attention, both in systematic outpatient evaluation and with specific treatment development to address this additional clinical symptomatology in AUD.
Prazosin, an α
1 adrenergic antagonist, reduces alcohol-related adrenergic hyperactivity and excessive drinking in laboratory animals (
10,
11). Prazosin also improves working memory and prefrontal cortical functioning under high uncontrollable stress in nonhuman primates (
12) and decreases stress-induced alcohol seeking in laboratory animals and in human alcohol-dependent patients (
13–
15). These findings suggest that prazosin’s effects in AUD may be specific to individuals who show acute alcohol abstinence–related distress, clinically manifested with at least some increases in alcohol withdrawal symptoms and associated craving, mood, and anxiety symptoms. Indeed, results of clinical trials of prazosin’s efficacy in AUD have been mixed, with some studies reporting improved drinking outcomes (
16,
17) and others showing no significant benefit in drinking outcomes in overall analyses, but with benefit in subgroups with higher blood pressure (
18,
19). Importantly, these clinical studies have varied significantly in participants’ recent alcohol use severity and pretreatment abstinence levels and have not reported assessments of alcohol withdrawal symptoms, and thus they have provided little information on whether alcohol withdrawal and abstinence symptoms may be a prognostic indicator of prazosin treatment in AUD (
20,
21).
Despite the growing need to identify moderators of clinical response in the treatment of AUD (
3,
20), systematic research on evaluating the relevance of prognostic clinical moderators of alcohol treatment response has lagged behind. As alcohol withdrawal symptoms are not observed in all patients with AUD entering treatment, and severity of the illness plays a significant role in its pathophysiology and chronic relapsing nature (
2), there is an urgent need to test whether alcohol withdrawal symptoms and related abstinence symptoms are a relevant factor in moderating AUD treatment outcomes (
3,
20). An ongoing problem in addressing this issue, however, is the lack of well-established thresholds for alcohol withdrawal and abstinence symptom assessments as they pertain to relapse risk, continued heavy drinking, and treatment failure. The Clinical Institute Withdrawal Assessment for Alcohol–Revised (CIWA-Ar) (
22) is a well validated and established measure that is widely used in assessing alcohol withdrawal syndrome for medical detoxification, but it is rarely used postdetoxification to assess residual withdrawal and associated abstinence symptoms to identify those at risk of relapse and treatment failure in outpatient settings.
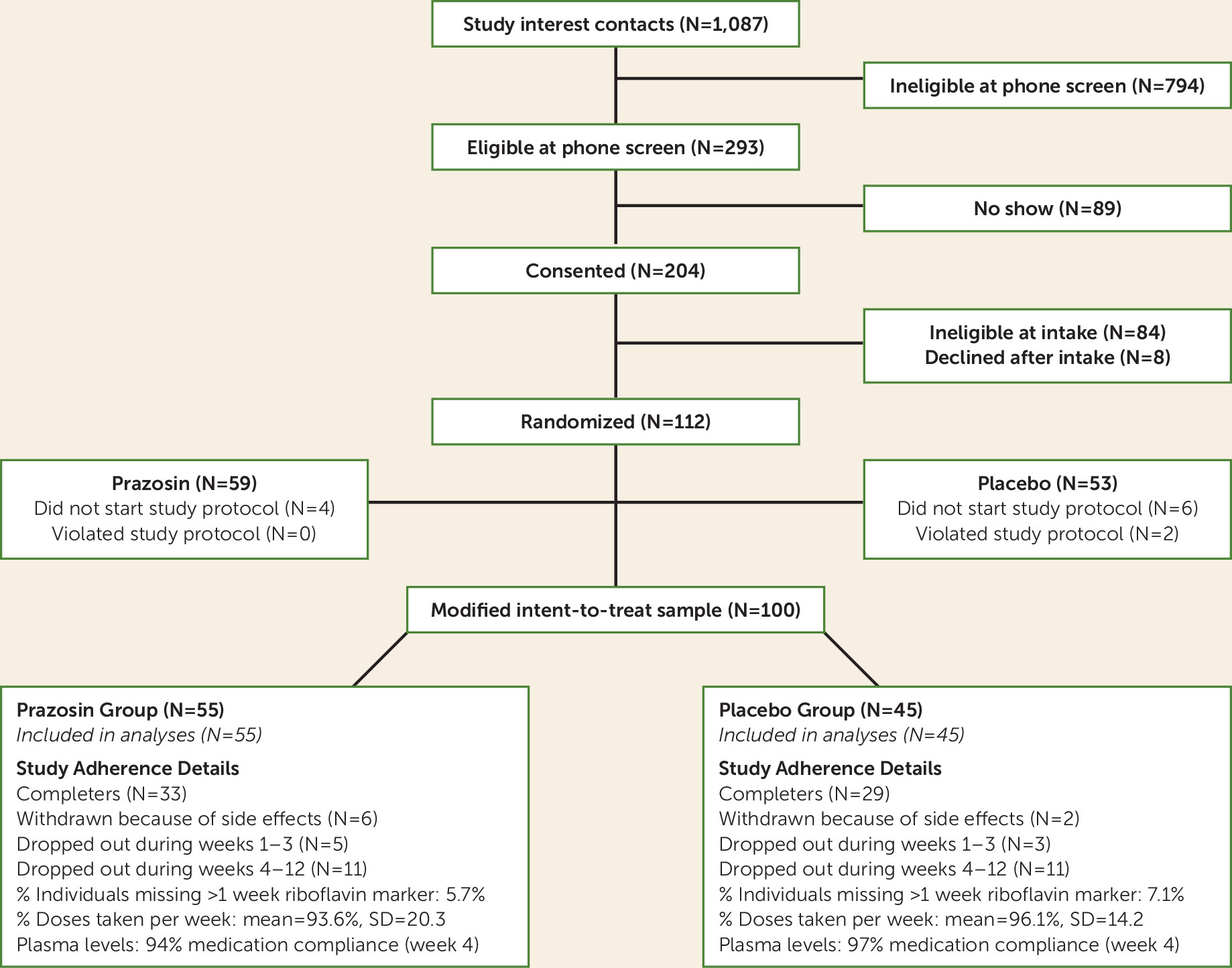
We conducted a proof-of-concept randomized clinical trial of prazosin in AUD to specifically evaluate the role of alcohol withdrawal symptoms in treatment-entering individuals in moderating the efficacy of prazosin on the primary drinking outcomes of heavy drinking days and any drinking days and the secondary drinking outcome of average drinks/day and clinical outcomes of alcohol craving, anxiety symptoms, mood symptoms, and sleep disturbances. As there is no specific established CIWA-Ar cutoff score to identify those with risk for relapse and treatment failure, we used CIWA-Ar scores as a continuous measure to assess the moderating influence of alcohol withdrawal symptoms on prazosin treatment effects. The primary hypothesis was that prazosin’s efficacy will interact with alcohol withdrawal symptoms to influence primary heavy drinking days and drinking days as well as average drinks/day and other secondary outcomes, such that prazosin’s efficacy in primary and secondary outcomes will be specific to individuals with high alcohol withdrawal symptom severity, and with no treatment benefit in those with low alcohol withdrawal symptom severity or no withdrawal symptoms. Also, as some data suggest that sympathetic hyperactivity, with higher baseline systolic or diastolic blood pressure, may moderate prazosin’s treatment efficacy, we conducted post hoc secondary analyses to explore these as moderator variables.
Discussion
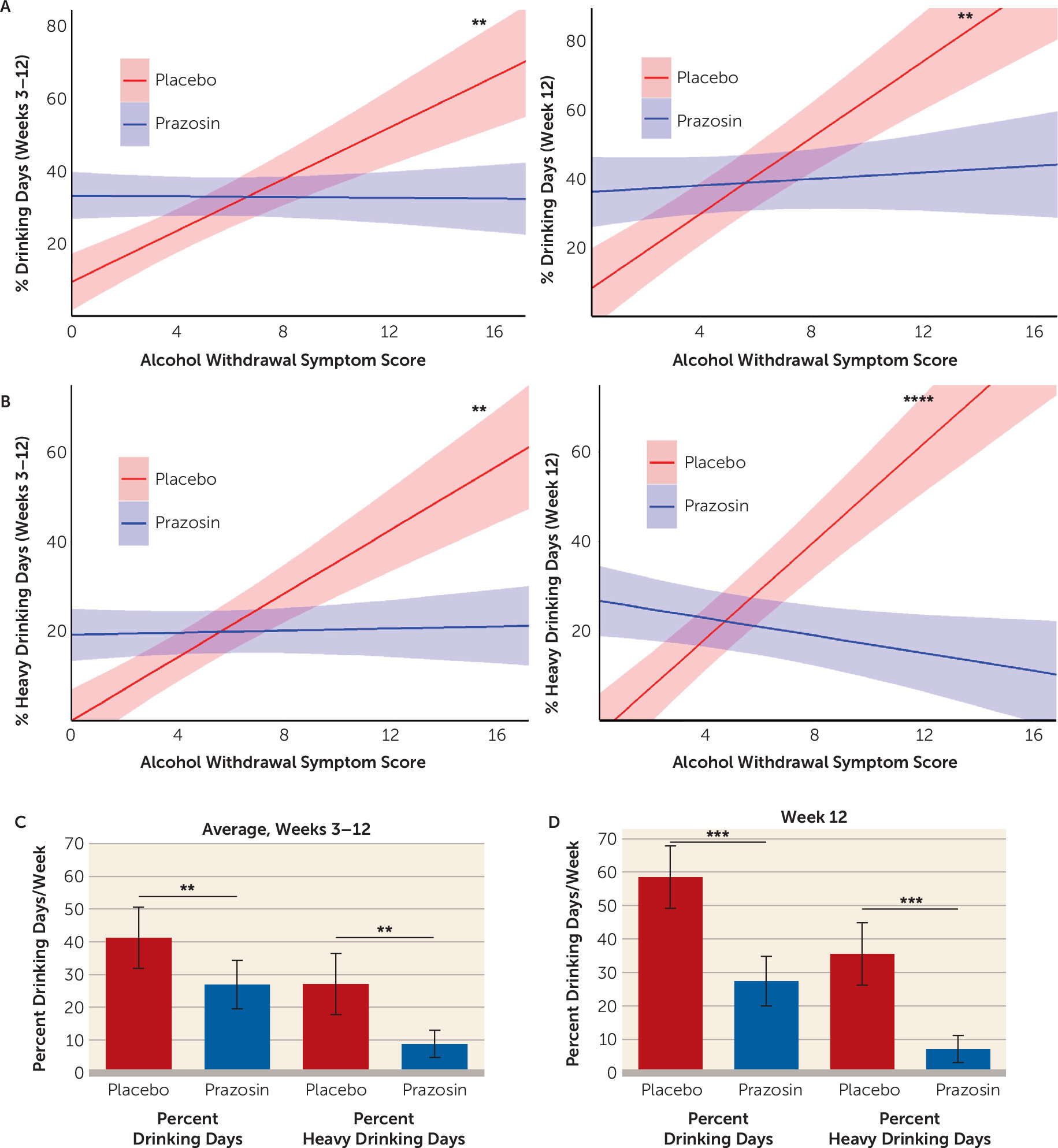
Our findings identify alcohol withdrawal symptoms as a significant moderator of prazosin’s treatment efficacy for primary and secondary alcohol use outcomes and for additional secondary outcomes of anxiety, depressed mood, and alcohol craving over the course of the trial. The findings indicate that the greater the baseline alcohol withdrawal symptom score, the higher the drinking outcomes in the placebo group, but this effect was reversed in the prazosin group. In direct comparison among individuals with high alcohol withdrawal symptoms (CIWA-Ar scores ≥3), there was a greater benefit of prazosin relative to placebo in reduction of heavy drinking, any drinking days, and average drinks/day over the course of the trial. In parallel, we found that the higher the alcohol withdrawal symptom severity, the greater the benefit of prazosin in reducing anxiety, depressed mood, and alcohol craving over the course of the trial. While higher baseline alcohol withdrawal symptom severity significantly influenced reduction in withdrawal symptoms during the study, these responses were not influenced by treatment assignment. Notably, those with no or low alcohol withdrawal symptom scores (two symptoms or fewer) showed no benefit from prazosin treatment. Together, the findings suggest that the presence of high alcohol withdrawal symptom severity at treatment entry may serve as a prognostic indicator of the potential benefit of prazosin treatment for alcohol use and related secondary outcomes.
Significant moderation of prazosin’s efficacy in reducing alcohol use outcomes by baseline alcohol withdrawal symptom severity may serve to explain, at least partially, the mixed findings from previous prazosin clinical trials for AUD (
16–
19). These studies enrolled individuals with significant variation in current alcohol use severity and in pretreatment abstinence and did not account for baseline alcohol withdrawal symptom severity, thereby including a heterogeneous group of individuals possibly varying in extent of alcohol withdrawal symptoms and other mood, anxiety, and craving symptoms indicative of greater AUD-related distress. In contrast, in the present study, we had no pretreatment abstinence requirement for enrollment. Thus, patients could have been in acute abstinence, with an increased likelihood of exhibiting alcohol withdrawal symptoms.
Notably, the sensitivity of these factors in affecting AUD treatment efficacy has been documented previously. For example, number of pretreatment abstinence days was found to be a significant factor affecting efficacy of injectable naltrexone in the treatment of AUD (
32), with greater number of days of pretreatment abstinence associated with better efficacy of naltrexone. Similarly, high alcohol withdrawal symptom severity was found to significantly affect the efficacy of gabapentin in the treatment of AUD (
33), and may also explain mixed results with gabapentin for alcohol use outcomes (
34,
35). Diastolic blood pressure has also previously been found to moderate prazosin’s efficacy in drinking outcomes (
19), a finding that was replicated here for the heavy drinking days outcome in our secondary exploratory analyses. These findings underscore the need to systematically evaluate alcohol withdrawal symptoms, blood pressure, and associated abstinence symptoms at treatment entry for their role as prognostic indicators of alcohol use outcomes.
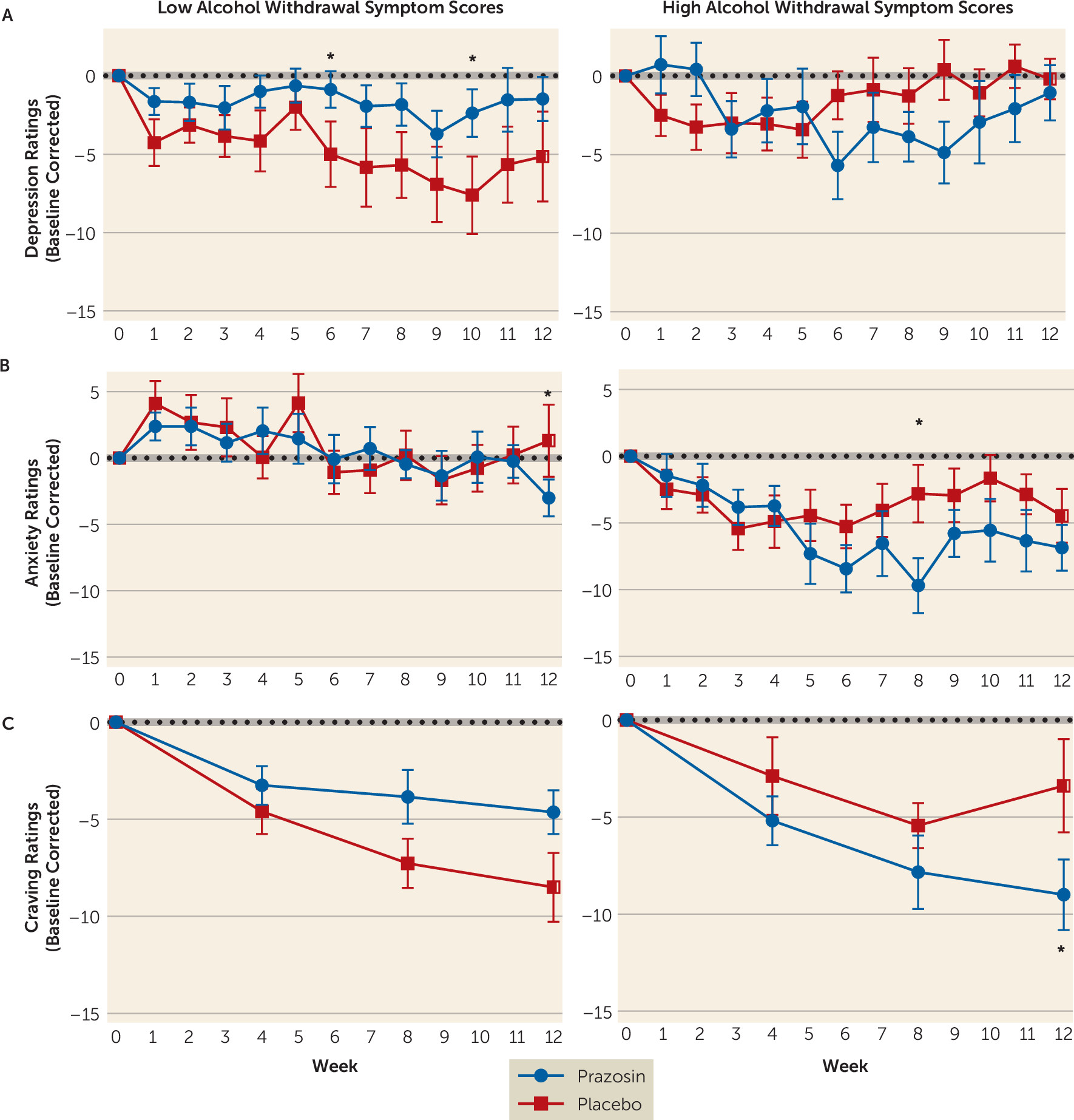
Consistent with the well-documented heterogeneity in clinical features of AUD, we found that placebo-treated individuals with no or low alcohol withdrawal symptoms successfully reduced their drinking after entering treatment and showed low levels of alcohol consumption throughout the trial. All participants received fishbowl contingency management as well as manualized, empirically validated 12-step counseling as behavioral support to promote treatment retention and recovery from AUD. Thus, it is not surprising that those with low alcohol withdrawal symptom scores and with less severe AUD were able to reduce and maintain lower drinking levels. Although the individuals with low or no alcohol withdrawal symptoms in the prazosin group were drinking more than those in the placebo group during the trial, the drinking outcomes during the full-dose period, after titration of prazosin, did not differ from each other. These findings underscore the importance of assessing alcohol withdrawal symptoms as a prognostic indicator of withdrawal and stress-related abstinence symptoms at treatment entry, which could potentially identify those individuals who may be responsive to behavioral treatments, while others with higher alcohol withdrawal and abstinence symptoms might require additional pharmacotherapy treatment options.
An important aspect of our results is that prazosin’s efficacy in individuals with high alcohol withdrawal symptoms was observed after participants achieved the full dosage of medication and progressively over the course of the trial, and not during the early weeks. These findings suggest that prazosin’s efficacy may not be due to its effects on the sympathetic hyperarousal associated with locus coeruleus hyperactivity during acute alcohol withdrawal, as noted in some previous work (
10,
11). In fact, we found that the prazosin group showed higher heart rate and (nonsignificantly) lower systolic blood pressure overall during the trial relative to the placebo group, but prazosin treatment did not have an impact on alcohol withdrawal symptoms and sleep problems during the trial. These data suggest a divergence of prazosin’s effects on sympathetic hyperarousal relating to acute withdrawal and its effects on anxiety, mood, craving, and alcohol use outcomes, with the former relating to prazosin’s effects on locus coeruleus pathways and the latter likely being associated with its effects in reducing adrenergic hyperactivity in forebrain cortico-striatal circuits that regulate stress, reward, and behavioral choices (
4,
9,
12,
14).
Higher alcohol withdrawal symptom severity has previously been associated with heavier drinking (
6,
36), as seen in the present sample at baseline and in the placebo group over the 12-week treatment period, which was reversed by prazosin in this study. As expected, we also found that higher alcohol withdrawal symptom scores were associated with greater stress-related symptoms of anxiety, depression, alcohol craving, and alcohol intake at treatment entry. These clinical symptoms pose a significant challenge to AUD treatment success, and there is growing acknowledgment of the need to address alcohol withdrawal symptoms and related abstinence symptoms that jeopardize alcohol recovery and increase relapse risk (
3,
4). Notably, we found significant effects of prazosin relative to placebo in reducing alcohol craving, anxiety, and mood symptoms over the course of the trial. Because chronic alcohol use exacerbates these symptoms, it is possible that prazosin’s effect of significantly reducing drinking outcome measures in individuals with higher alcohol withdrawal symptoms led to concomitant reductions in these secondary symptoms. However, previous work indicates that stress and negative mood increase alcohol craving, which in turn increases lapses and relapse risk (
26,
37,
38), and that prazosin treatment reduces such stress-induced alcohol craving, negative emotions, and anxiety (
13,
39). These findings support the speculation that prazosin’s benefits may be mediated by its effects on the high craving, negative mood, and anxiety that are clinically observed in those with greater alcohol withdrawal symptom severity. Further research is needed to understand prazosin’s effects on these abstinence symptoms as they relate to alcohol use outcomes.
This study had some limitations. First, while women were included, they made up only one-third of the sample, and although gender was controlled for in the analyses, there was not adequate power to assess gender differences in treatment response. Second, no objective biological measure of alcohol intake was used as a primary outcome measure. Third, while medication adherence documented by urine and plasma levels was good and was comparable across treatment groups, plasma levels were assessed only once, at weeks 4–5, and no blood levels were available at later points in the trial. Finally, although the between-group difference was not significant, more individuals in the prazosin group were withdrawn because of side effects (N=6) than in the placebo group (N=2), and this effect will need further attention in future studies.
Despite these limitations, the present findings address an important clinical problem in AUD treatment, namely, heterogeneity of treatment responses and modest efficacy of currently available treatments. Our findings support the need to systematically assess alcohol withdrawal symptoms and related abstinence symptoms at treatment entry as a prognostic indicator of disease severity, while also suggesting the utility of alcohol withdrawal symptom severity as a key moderator of treatment response for a widely available, safe, and well-tested therapeutic agent such as prazosin. Although this is a proof-of-concept study and thus requires specific replication in those with high alcohol withdrawal and abstinence symptoms, the results shed new light on the widely implementable clinical diagnostic measure of alcohol withdrawal and abstinence symptoms, which may be used as a prognostic indicator and precision-medicine tool to address potential treatment failure and the need for pharmacotherapies like prazosin to improve alcohol treatment outcomes.