Compulsive behaviors are prominent across a wide range of psychiatric disorders, notably in compulsive behavior spectrum disorders (e.g., obsessive-compulsive disorder [OCD]), and are associated with marked impairment and staggering societal burden (
1). In all their diverse manifestations, compulsive behaviors can be characterized as a failure of flexible goal-directed behavior to override habitual behaviors (
2). While these behaviors may serve a function initially (e.g., short-term reduction of distress, perception of greater certainty), they typically become highly aversive and are recognized as contrary to life goals.
“Dual-system” theories of instrumental action (
3) posit that the decision to perform an action is determined by the balance between the habitual system, which supports inflexible stimulus-response associations that are “stamped in” through repetition, and the goal-directed system, which supports flexible application of knowledge and motivational contingencies. Neuropsychological executive deficits in patients with OCD (e.g., deficits in set shifting, reversal learning, and response inhibition) are potentially consistent with a core imbalance in the ability to flexibly implement goal-directed behavior and override habit (
4,
5). Recent studies have more specifically suggested that, in the context of habit formation, goal-directed behavior is impaired in patients with compulsive behaviors (e.g.,
6,
7).
Both animal and human findings converge on alterations within cortico-striato-thalamo-cortical (CSTC) circuits as a key factor in compulsive behaviors and highlight one key structure in particular—the orbitofrontal cortex (OFC). However, ambiguity persists regarding the OFC’s role as either a driver or an overrider of compulsive behaviors. The dominant neurocognitive account of imaging findings in OCD suggests that greater activation of a “direct,” excitatory, feed-forward CSTC pathway relative to an “indirect,” inhibitory pathway results in overall hyperactivation of CSTC circuits (
8,
9). This includes hyperactivity in the OFC at rest and during symptom provocation, one of the best-replicated neuroimaging patterns in OCD patients (
10). However,
decreased activation of the OFC is observed in OCD during some cognitive tasks (
10), consistent with the OFC’s critical role in promoting goal-directed behavior. Furthermore, a large meta-analytic effort failed to identify consistent cortical thickness abnormalities in the OFC (
11). Such inconsistencies likely reflect the inherent imprecision of correlational methodologies, functional MRI (fMRI) susceptibility artifacts within the OFC, heterogeneity among patients, and the OFC’s diverse and nonstatic role within CSTC loops, supporting a broad array of behavioral responses via pathways within and beyond CSTC circuits.
The correlational nature of both neural and neuropsychological findings in patients hinders the differentiation of neural patterns that are causal in producing compulsive behaviors from those that are effects or “epiphenomena.” In contrast, animal research techniques afford exquisite experimental precision in modulating neural circuitry relevant to compulsive behavior and measuring downstream behavioral effects. Such studies have demonstrated a causal role for the OFC in modulating compulsive behaviors and habit, yet the direction of effect remains ambiguous. Optogenetic studies in rodents (
12) have established that brief but repeated activation of medial OFC-to-ventral striatum glutamatergic synapses induces excessive grooming behavior in mice (
10,
13). Optogenetic disruption of activity in a nearby ventromedial prefrontal cortex (vmPFC) region blocked habit formation and expression in rats (
14,
15), further suggesting that the OFC helps drive habit and compulsive behaviors. However, the OFC plays an equally critical role in promoting goal-directed behavior. Consequently, chemogenetic inhibition of the OFC can disrupt goal-directed behavior in mice, while optogenetic OFC activation increases goal-directed behavior (
16) and inhibitory control over a habitual grooming response (
17).
The direct relevance of these opposing mechanisms to humans who engage in problematic compulsive behaviors, and the ability to harness these insights in the development of novel treatments, remains unclear without a direct parallel test of causality in humans to explicate the OFC’s directional role. This is particularly critical given that cross-species comparisons suggest important discrepancies between findings with regard to the OFC’s functions and functional organization that make rodent homologues an imperfect approximation of humans (
18). Theta burst stimulation (TBS) (
19), a form of transcranial magnetic stimulation (TMS), has been used for noninvasive causal manipulations to acutely, experimentally manipulate brain function in two opposing directions. TBS may create a 50- to 60-minute window of increased (with intermittent “bursts” of stimulation; iTBS) or decreased (with continuous pulses; cTBS) neural activity using a potent, very brief (40–120 seconds total) approach. Although the majority of TBS studies have targeted the motor cortex, data supporting the safety, tolerability, and target engagement of iTBS and cTBS targeting the OFC and frontopolar cortex (FPC) have recently begun to accumulate in healthy volunteers (
20) and clinical populations (
21).
However, relative to animal models, limitations exist in the spatial and circuit-level precision of currently available methods for noninvasive stimulation in humans, which complicates hypothesis tests, given substantial functional heterogeneity within the OFC and neighboring FPC regions (
22,
23). To overcome this, we used immediate post-TBS completion of a “habit override” task, thereby isolating the specific OFC functional mechanism that we aimed to augment, effectively aiming to “exercise” clinically relevant habit override abilities directly after stimulation.
We used a single session of iTBS and cTBS to test competing hypotheses relevant to the development of a comprehensive pathophysiological model of compulsive behaviors in patients—namely, that increased
or decreased OFC activation might be beneficial, enhancing patients’ capacity to override compulsive behaviors. Given spatial diffusion of the TMS signal, with the goal of modulating the OFC at large, we selected a left OFC/FPC target (left Brodmann’s area [BA] 10, at its orbital junction with BA 11). This target was selected both in order to mimic the methods of previous TBS studies that provided crucial validation for the safety, tolerability, and effectiveness (i.e., target engagement) of modulating orbital areas using a standard TMS coil (
24,
25) and for consistency with a previous TMS study in OCD patients that also targeted the left OFC/FPC and showed clinical benefit (
26). To focus the effects of the stimulation and thereby enhance both the theoretical and the clinical implications of the findings, TBS was followed immediately by behavioral practice in a habit override task. This provided a behavioral context for the OFC/FPC manipulation and allowed us to ask the more specific question of what role the OFC plays in promoting—or interfering with—the acquisition of a highly clinically relevant skill (overriding avoidance habits). The primary study outcomes were cerebral blood flow in the OFC (target engagement) and participants’ acute capacity to override compulsive behaviors during an idiographic stressful laboratory probe, measured on the same day (90 minutes after TBS) and, in exploratory analyses, at 1-week follow-up. The study’s goal was to establish the first experimental evidence in humans for a mechanistic model in order to inform further experimental work and, ideally, the eventual development of novel mechanistic treatments involving synergistic biological-behavioral pairings (
27).
Methods
Study Design
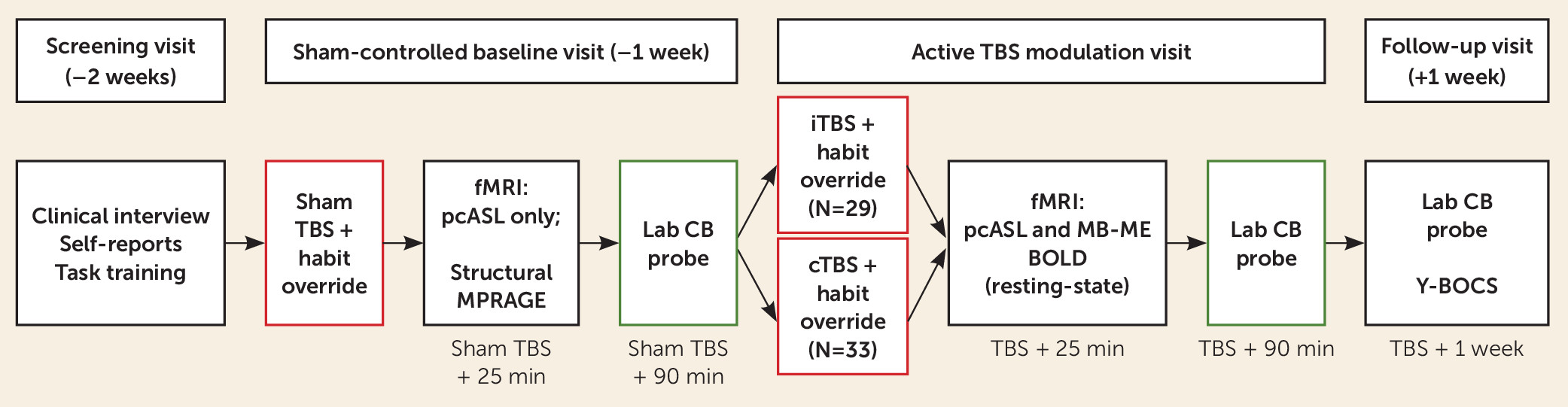
The study design (
Figure 1) included parallel-arm randomization and patient and assessor blinding. Because our primary comparisons were for iTBS versus cTBS, all participants were allocated to one of these two arms, using a between-group design to eliminate crossover and practice effects. However, we used a baseline single-blind sham TBS lead-in to create parallel procedures at the baseline assessment. As detailed further below and in
Figure 1, each of the two principal visits (sham-controlled baseline and active TBS) included pre-TBS habit acquisition, TBS (sham or active), habit override training, fMRI assessment of target engagement, a 45-minute break to allow acute TBS effects to dissipate, and a postacute (90 minutes after TBS) laboratory probe of compulsive behaviors. A 1-week follow-up assessment included the laboratory probe of compulsive behaviors as well as a repeat clinical interview. Clinical assessors were successfully blinded to treatment allocation (see the
online supplement).
Participants
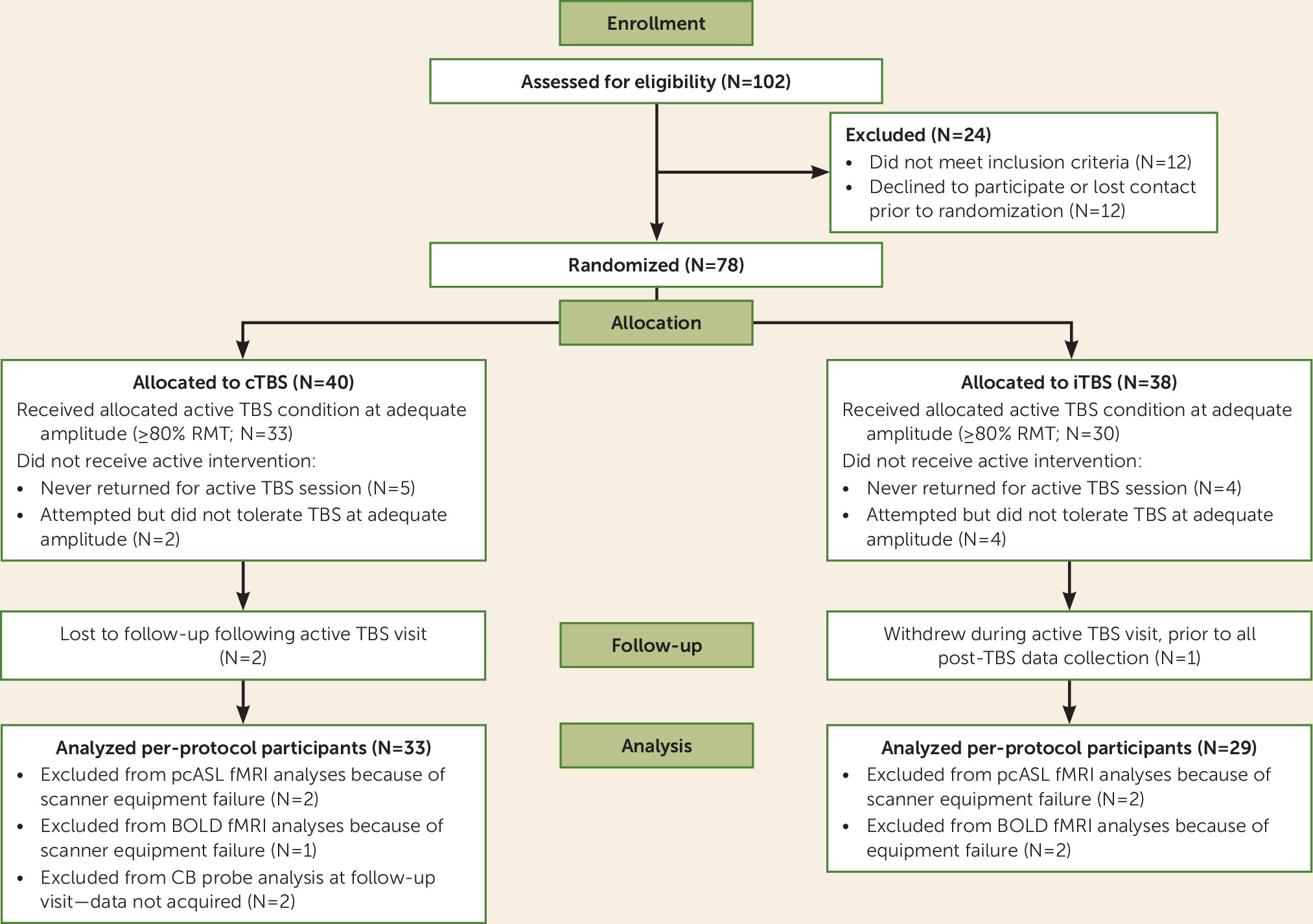
Seventy-eight adults (ages 18–55) underwent randomized assignment and completed all baseline assessment procedures, including the single-blind sham TBS session; of these, 69 participants returned to attempt active TBS (
Figure 2). Two clinical inclusion criteria (see the
online supplement for full details of inclusion and exclusion criteria) were used to identify clinically meaningful forms of compulsive behavior (i.e., both DSM-5 obsessive-compulsive and related disorders and “diagnostic orphans” with similar problematic compulsive behaviors): a score greater than one standard deviation above the published mean of healthy control subjects on at least one self-report scale of compulsive behaviors, and clinically significant compulsive behaviors per clinician rating on the compulsions subscale of the Yale-Brown Obsessive Compulsive Scale, 2nd edition (
28). According to baseline diagnostic interviews, these transdiagnostic inclusion criteria yielded a sample with an average of 2.22 DSM-5 obsessive-compulsive and related disorders (see
Table 1 for sample characteristics).
TBS
After determining resting motor threshold (RMT) through standard procedures (
29), the TMS coil (a figure-8 MCF-B65; MagVenture, Denmark) was positioned stereotaxically over a navigational-system-identified left OFC/FPC anatomical target (see the
online supplement for details on neuronavigation).
We followed clinical TMS conventions and adapted published protocols (
25) to increase tolerability and comfort and achieved high compliance with TBS procedures, as observed in other clinical populations (
21). This included a stimulation ramp-up block (600 pulses beginning at 0% and ramping up gradually over the entire block, such that few pulses were delivered at an efficacious amplitude; see the
online supplement), followed by the active block (600 pulses), which was delivered at the target amplitude of 110% of RMT (as in previous OFC/FPC cTBS studies [
24]) or at the maximum amplitude tolerated by the participant during ramp-up. Based on findings from TBS clinical studies targeting the prefrontal cortex (PFC) (
30,
31) and electric field simulations (see the
online supplement), a minimum tolerated amplitude of 85% of RMT was considered adequate for efficacious stimulation, and thus participants who completed the active TBS block at ≥85% of RMT (N=59 at 110%; N=4 at 86%−104%) were included in all analyses. For these participants, the TBS pulse amplitude as a percentage of the maximum stimulator output was 55.8% (SD=6.6; range=38−68) for cTBS and 52.6% (SD=5.9; range=41−65) for iTBS.
In all conditions, pulses were applied in a theta burst pattern (bursts of three stimuli at 50 Hz repeated at 5 Hz frequency) using a MagPro X100 stimulator (MagVenture). iTBS consisted of 20 trains, each lasting 2 seconds with intertrain intervals of 8 seconds, for a total of 192 seconds. cTBS consisted of one continuous train of 40 seconds. Sham TBS (delivered to all participants at the baseline session) utilized the participant’s allocated active TBS protocol over the same target, run at 20% of maximum stimulator output to provide a consistent absolute level of scalp stimulation and therefore sensation (32%−57% of RMT, below the threshold expected to induce reliable cortical effects). See Figure S2 in the online supplement for a representative computer simulation of the distribution of the electric field induced by TBS in the cortex.
Habit Override Training
During the expected window of TBS modulation (on the active TBS day, and during the same window on the sham TBS day), a habit override training task was administered, modeled after previous OCD research (
6) (see Figure S1 in the
online supplement). In brief, in
habit acquisition (delivered prior to TBS), participants were instructed that their goal was to avoid receiving shocks to the left and right foot by pressing appropriate buttons whenever a conditioned cue appeared. Across 480 trials (3 seconds per trial, 24 minutes total), participants overlearned these avoidance behaviors. In the subsequent
habit override task (240 trials, 3 seconds per trial, 12 minutes total), which was administered 15–30 minutes after TBS, the same pairs of cues were presented, but one of the two overlearned habits was “devalued.” A written and verbal instruction was provided informing participants which one of the two electrodes had been disconnected and stating that they should attempt to resist the relevant “devalued” avoidance response (i.e., override that habit), while continuing the remaining “valued” habit. As detailed in the
online supplement, performance patterns conformed to expectations and suggested that overriding the shock-avoidance habit was challenging for participants.
fMRI Assessments
Scanning was performed in a Siemens 3-T Prisma Scanner, beginning approximately 25 minutes after TBS (sham or active TBS), immediately following completion of the habit override task, allowing all fMRI data collection to occur within the anticipated window of acute TBS modulation. During a 4-minute resting-state block, collected both at the baseline (post–sham TBS) and post–active TBS visits, two-dimensional pseudo-continuous arterial spin labeling (pcASL) was acquired (TR=4.9 seconds; TE=16 ms; 25 4-mm slices with 1-mm interslice gap; 3.28×3.28 mm voxels; labeling duration=1800 ms; postlabeling delay=1800 ms; 25 pairs of labeled/control acquisitions) and preprocessed as recommended using SPM’s ASL perfusion MRI signal processing toolbox (ASLtbx; details are provided in the
online supplement). pcASL provides stable, interpretable quantification of absolute brain activation (cerebral blood flow) (
32), with adequate test-retest reliability and convergent validity with positron emission tomography (
33,
34). A broad prefrontal/anterior mask was defined by all voxels within the Montreal Neurological Institute template with y≥22. A voxel-wise search was then performed within this mask for clusters differentially modulated by TBS condition (voxel-wise p=0.005, map-wise p<0.05).
During an additional 7-minute resting-state block, blood-oxygen-level-dependent (BOLD) data were acquired with a multiband/multiecho (MB-ME) sequence optimized to capture BOLD signal in ventral/OFC regions (TR=1 second; TE=13.2, 38.76, and 64.32 ms; multiband factor=5; flip angle=49°; matrix=68×68; resolution=3-mm isotropic voxels; 40 oblique slices; 480 volumes), which has been found to substantially reduce susceptibility artifact and increases BOLD signal-to-noise ratio by up to 80% in the OFC (
35–
37). Given budgetary and scanner time constraints, the MB-ME sequence was acquired only at the second MRI visit, after active iTBS or cTBS. At the first session (following sham TBS), a high-resolution structural scan (using MPRAGE [magnetization-prepared rapid acquisition with gradient echo]) was obtained instead, for input to the TMS neuronavigation system. We observed post hoc that this customized MB-ME sequence was indeed less susceptible to signal dropout in orbital areas relative to pcASL; thus, this secondary measure of left OFC target engagement was used strictly to confirm whether differential modulation extended to anatomically defined left BA 47 and 11, where pcASL signal dropout was observed.
After standard preprocessing in AFNI (details are provided in the
online supplement), to quantify the intensity of spontaneous brain activity during the resting state (
38), AFNI’s
3dRSFC tool was applied to quantify fractional amplitude of low-frequency (0.01–0.1 Hz) fluctuations (fALFF), a putative index of the absolute level of resting activation following TBS. Mean fALFF within a priori target regions (left BA 47 and 11, defined within AFNI’s TT_Daemon atlas) was extracted for each individual and compared across the two TBS conditions with an unpaired t test.
Laboratory-Triggered Compulsive Behavior Assessment
A laboratory probe of compulsive behavior vulnerability was completed at the baseline sham visit and the active TBS visit (90 minutes after TBS) and, as an exploratory probe of the durability of TBS’s impact, repeated at the 1-week follow-up visit. We idiographically designed, collaboratively with the participant, a laboratory task involving a triggering object, image, or scenario that corresponded to compulsive behaviors typical to the participant. The idiographic trigger was presented to the participant alone in a controlled laboratory environment, along with the means to engage in typical compulsive behaviors (e.g., hand sanitizer/sink; mirror for skin picking; necessary media for reassurance seeking/checking). To assess ability to voluntarily override compulsive behaviors, participants were instructed to confront the trigger and then resist or decrease compulsive behaviors to the extent possible, and were then left alone for 5 minutes. Posttask ratings of urges (0–100), effort (0–100), and success (i.e., total estimated duration of time spent engaged in compulsive behaviors) were collected. The ratings were correlated with scores on the compulsions subscale of the gold-standard Yale-Brown Obsessive Compulsive Scale (see Table S1 in the online supplement), suggesting that we captured clinically relevant symptoms, but on an immediate time scale suitable for sensitively capturing an acute clinical impact.
See the online supplement for details on our analytic strategy, including the rationale for the per-protocol analyses.
Results
Modulation of the OFC/FPC Target
Tolerability.
The two conditions were equally well tolerated; 94% (N=33/35) of participants tolerated cTBS stimulation at ≥85% of RMT, and 88% (N=30/34) tolerated iTBS (Fisher’s exact test, p=0.43) (further details are provided in the online supplement). No serious or unanticipated adverse events occurred.
pcASL.
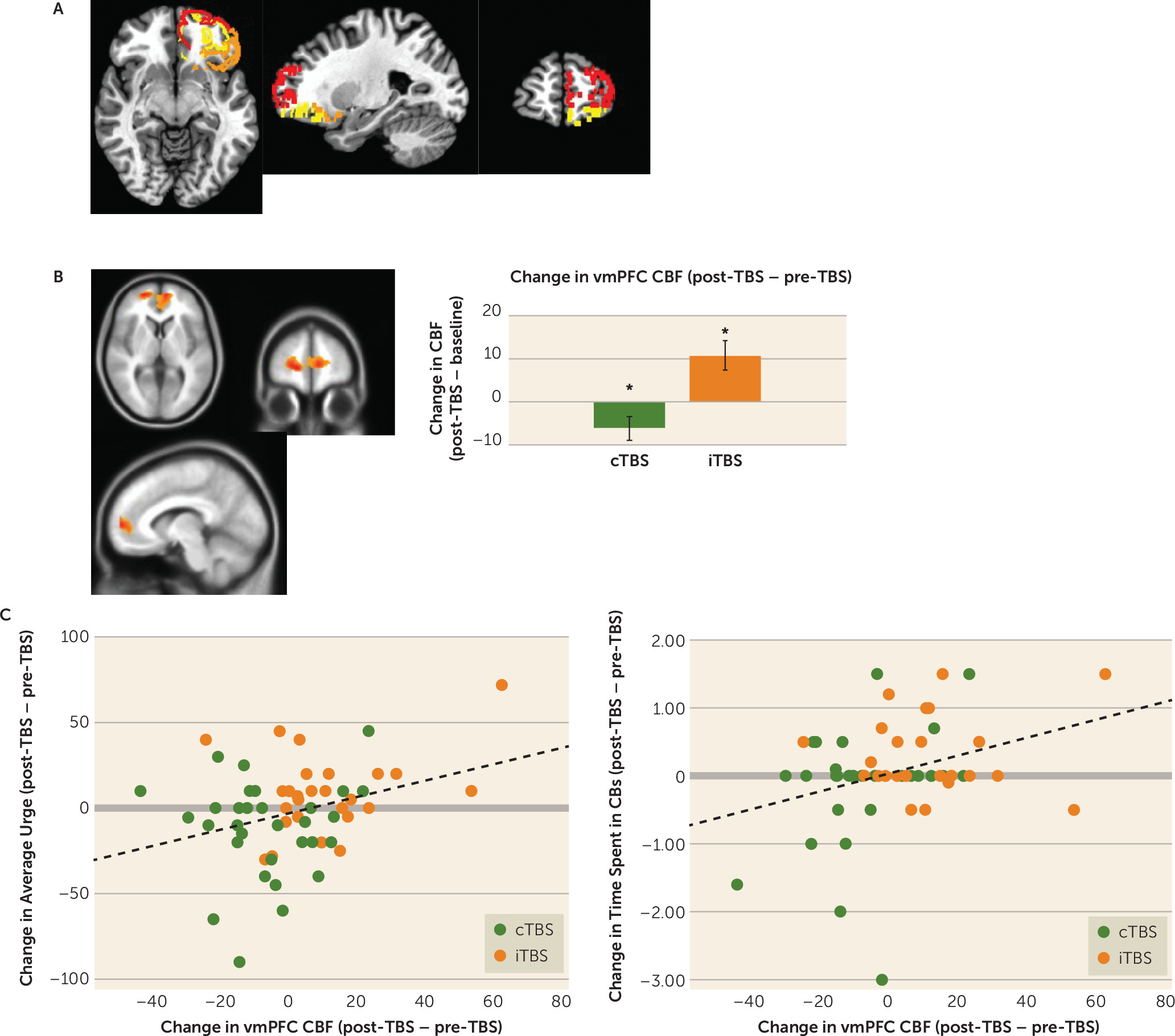
The pcASL sequence acquired both before (at the baseline/sham TBS visit) and after active cTBS/iTBS showed that TBS condition moderated pre- to post-TBS changes in cerebral blood flow in the target area of BA 10 and a surrounding vmPFC area (voxel-wise uncorrected p<0.005, N=736 voxels within PFC mask; map-wise corrected p<0.01; partial eta-squared=0.21) (
Figure 3B), with the expected direction of modulation observed for both cTBS (decrease: post hoc paired t=−2.17, df=30, p=0.038; d=0.39) and iTBS (increase: paired t=3.14, df=26, p=0.004; d=0.68).
fALFF.
Lower fALFF was observed following cTBS, relative to following iTBS, in the left BA 47 (t=−2.02, df=57, uncorrected p=0.048; Cohen’s d=0.53) but not the left BA 11 (t=0.27, df=57, uncorrected p=0.79; d=0.07).
Acute Effect of OFC/FPC Modulation on Compulsive Behaviors
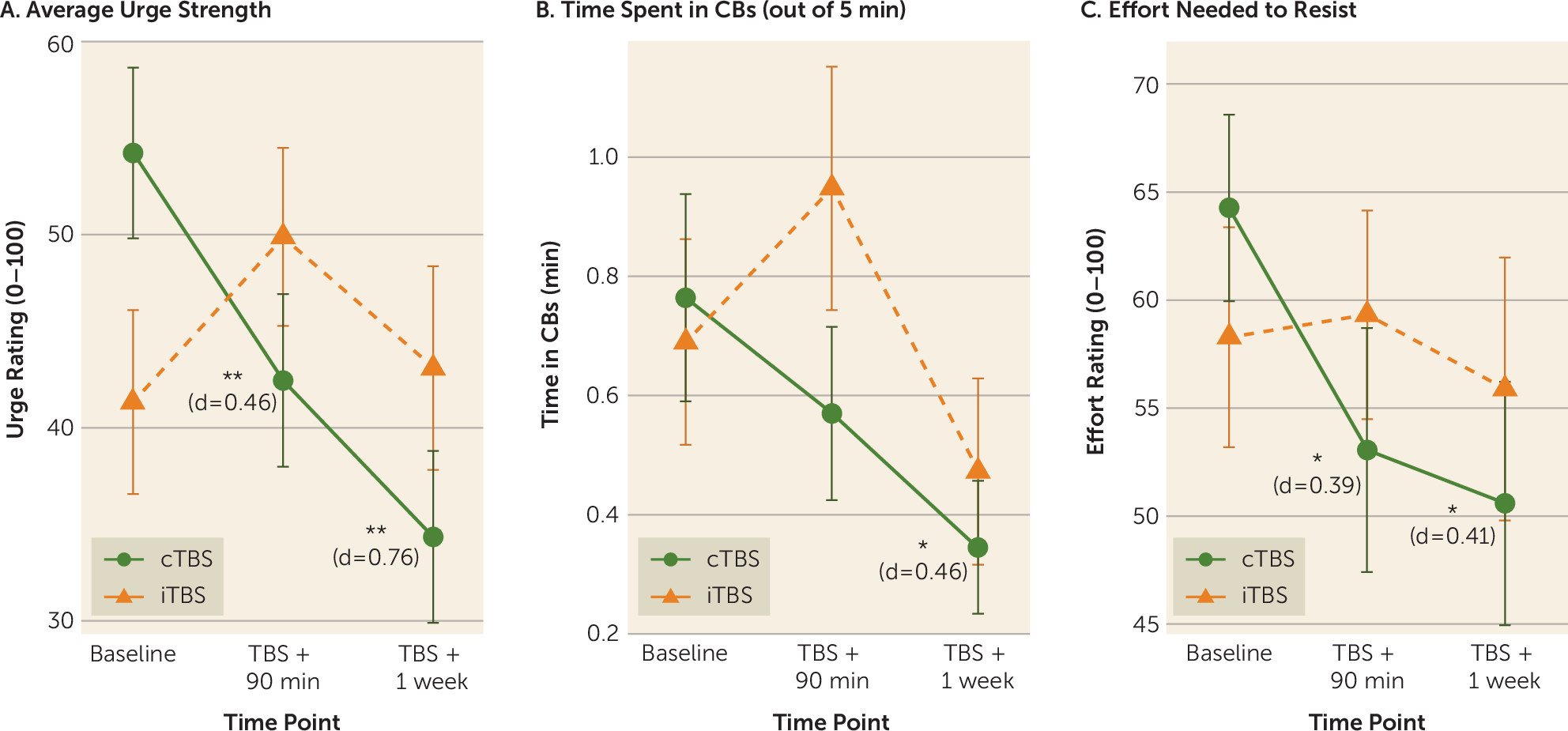
In linear mixed models focusing on the two primary time points (baseline and active TBS + 90 minutes), group-by-time interactions were observed in laboratory measures of compulsive behavior urge strength (β=20.32; t=3.28, df=60, uncorrected p=0.002, false discovery rate corrected [FDR] p=0.006; effect size r=0.39) and time spent engaged in idiographic compulsive behaviors (β=0.45; t=2.45, df=60, uncorrected p=0.017, p
FDR=0.026; effect size r=0.30). For effort needed to resist compulsive behaviors, the interaction was nonsignificant (β=12.25; t=1.71, df=60, uncorrected p=0.093, p
FDR=0.093; effect size r=0.22), although a significant pre- to post-TBS reduction was observed in the cTBS group (p=0.032; d=−0.39) but not the iTBS group (p=0.85; d=−0.04). Across all three indices (
Figure 4), the pattern of change suggested that cTBS produced pre- to post-TBS improvements, while indices were unchanged or (nonsignificantly) worsened after iTBS.
Across individuals, larger cerebral blood flow reductions in the vmPFC functional region of interest (
Figure 3C) from pre- to post–active TBS correlated with larger reductions in urge strength (r=0.32, uncorrected p=0.015, p
FDR=0.045) and time spent in compulsive behaviors (r=0.32, uncorrected p=0.015, p
FDR=0.023), but not effort (r=−0.02, uncorrected p=0.88, p
FDR=0.88).
In exploratory within-group analyses probing the durability of change at the 1-week follow-up, in the cTBS group, significant decreases from baseline were observed in urge strength (paired t=−3.39, df=30, uncorrected p=0.002, p
FDR=0.006; d=0.76), time spent in compulsive behaviors (paired t=−2.51, df=30, uncorrected p=0.018, p
FDR=0.027; d=0.46), and effort needed to resist (paired t=−2.28, df=30, uncorrected p=0.030, p
FDR=0.03; d=0.51) (
Figure 4), while no significant enduring effects were found for iTBS (uncorrected p values >0.31; d values, 0.07–0.25).
Discussion
In this study, we found that a single session of intermittent or continuous TBS was successful in 1) increasing and decreasing (respectively) cerebral blood flow within the left OFC/FPC (BA 10) and surrounding vmPFC areas (
Figure 3), and 2) modulating the left ventrolateral OFC (BA47), according to a secondary index of spontaneous brain activity (fALFF). Furthermore, the single 40-second session of cTBS, paired with the context of behavioral training in habit override, reduced acute markers of clinically relevant compulsive behaviors measured in the laboratory, relative to iTBS (
Figure 4). Acute in-laboratory behavioral improvements in the cTBS condition endured at 1-week follow-up, with medium to large effect sizes. Although this suggested possible durability that exceeded what would typically be expected following a single session of rTMS delivered in isolation (i.e., without a paired behavioral manipulation), effects of the brief, single-session manipulation did not extend to clinical symptom scales that measure more generalized and enduring symptomatic patterns (see the
online supplement).
Our findings add to a growing body of published studies suggesting that brief, noninvasive TBS procedures can have an impact on resting or baseline activation in the OFC/FPC and nearby ventral medial PFC areas—even when using a standard figure-8 TMS coil (e.g.,
24,
25). Notably, consistent with expected spatial diffusion of TMS signal, evidence of target engagement extended to a broader medial PFC area, suggesting modulation of a wider PFC network with the potential to influence a broad range of functions.
Neuroanatomical models of OCD, which center broadly on CSTC circuits, have provided important groundwork, but progress has been hindered by the inherent limitations of correlational (e.g., cross-sectional group comparison) designs in human studies. Here we circumvented that limitation by performing causal TBS studies, which led to findings consistent with animal experiments (e.g., optogenetic stimulation of the OFC produced excessive grooming in mice [
10,
13]). By decreasing OFC/FPC activity in compulsive behavior patients via cTBS, we posit that we may have pulled a chronically hyperactive OFC state down from the “ceiling,” opening a window of opportunity for more reflexive OFC activation and adaptive, goal-directed learning to occur. Within this window of opportunity, behavioral training may provide critical opportunities to make clinical effects of neuromodulation more efficient, stronger, and/or more enduring. Similarly, a multisite randomized controlled trial of “deep TMS” targeting the medial PFC/anterior cingulate (
39) suggested that active stimulation outperformed sham stimulation in reducing OCD symptoms, when both active and sham TMS were paired with the context of a prestimulation behavioral manipulation (brief exposure to a trigger). This led the U.S. Food and Drug Administration to approve marketing of deep rTMS to OCD patients, provided the behavioral exposure context protocol is specifically followed, under the assumption that this behavioral framing may be an influential factor. “State-dependent” neuromodulation (
40–
42) may thus play a key role within emerging theory-driven, synergistic biological-behavioral treatments (
27).
This study had several limitations. It was powered only to detect medium effect sizes or larger; yet several such effects were detected. Per-protocol analyses, which measure the effect of
using (i.e., tolerating) a given treatment, have less real-world clinical relevance and greater possibility of selection bias than intent-to-treat analyses, which measure the effect of merely being assigned/randomized to a treatment; well-powered studies should confirm these initial findings using an intent-to-treat principle. Emerging motor physiology work suggests that the directionality of TBS effects is affected by a complex interaction between RMT, cortical depth, and number of pulses (e.g.,
43), suggesting that future work would be optimized by a more idiographic and nuanced approach to dosimetry (e.g.,
44). Furthermore, neural target engagement was quantified as single-point averages reflecting spontaneous/baseline activation in a priori regions, whereas TBS effects on network-level activation and functional connectivity may be broad and dynamic (
45). Definitive experimental studies should include 1) both sham and cTBS treatment arms, with and without habit override training, to thoroughly assess key unanswered questions about necessary and sufficient intervention components; 2) larger samples permitting diagnosis-specific analyses, particularly in an effort to better delineate effects on relatively “compulsive” versus relatively “impulsive” behaviors (
46–
48); and 3) healthy control subjects, to assess whether procedures “normalize” neural and/or behavioral patterns. Such rigorous experimental studies will serve as essential precursors to facilitate optimized, mechanistically targeted treatment development.
Conclusions
We posit that by decreasing drive toward habitual and compulsive behaviors, down-regulation of the OFC/FPC (via cTBS) may provide a window of opportunity in which to enhance goal-directed behavior, after first “muting” OFC-mediated habit system interference. Our novel, fully automated behavioral training in habit override may have leveraged this acute brain state, creating a short-term benefit observed specifically on idiographic compulsive behaviors, which persisted for 1 week. Our study results advance an integrative picture of both neural and behavioral pathophysiological processes that contribute to compulsive behaviors. Future studies would benefit from inclusion of crucial experimental controls (e.g., sham TBS, cTBS without habit override) to clarify the role that behavioral context may play in leveraging an acute OFC/FPC state manipulation. With further extension of these initial experimental findings, this line of inquiry could represent a first step toward developing and clinically optimizing neuromodulation therapies (e.g., multisession synergistic biobehavioral treatments) that are well grounded in basic experimental science and leverage acute neuroplasticity to maximize clinical benefit.
Acknowledgments
The authors gratefully acknowledge Daniel M. Blumberger, M.D., Sabine Wilhelm, Ph.D., the study participants, and all members of the Clinical Application of Neuroscience lab for their contributions to this work.