Imaging Aβ.
The development of ligands that target the underlying neuropathology of AD, most notably Aβ and tau, has resulted in major advances in PET imaging in AD (
12–
20). A number of PET tracers that target Aβ were developed and are now used clinically in the diagnosis of AD (
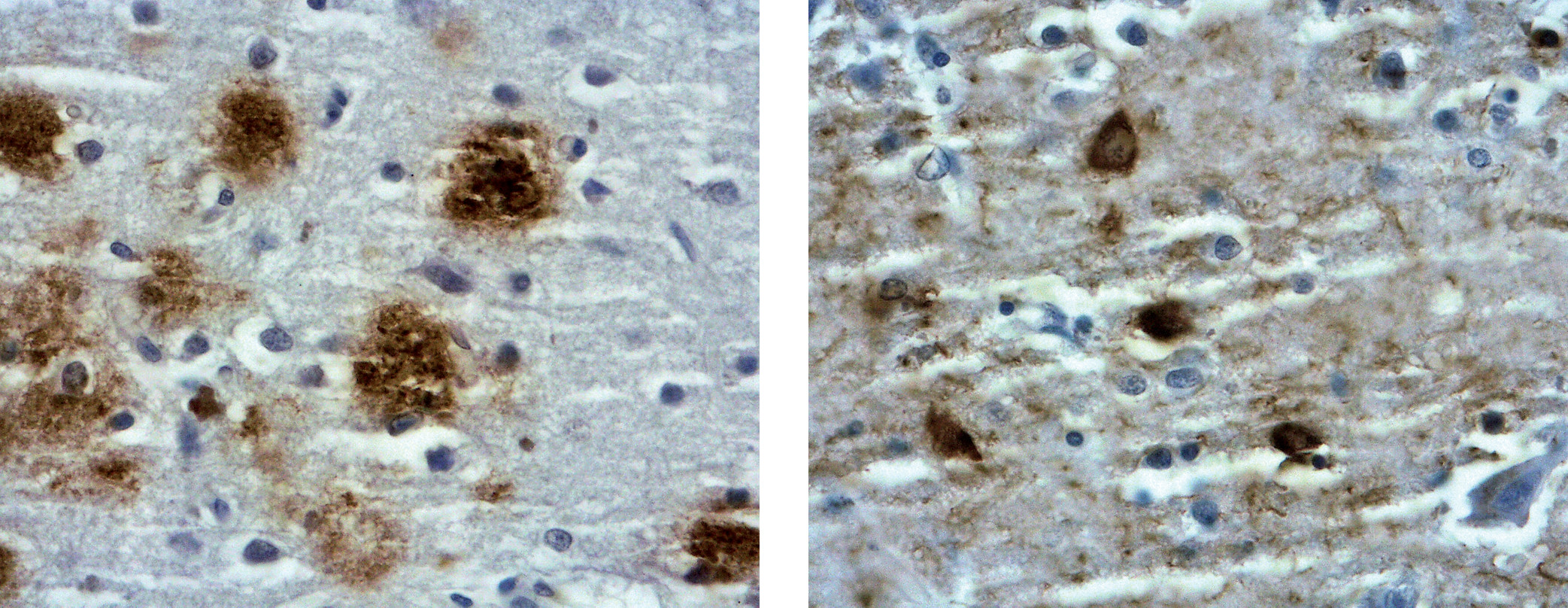
Figure 3). Pittsburgh compound B (PiB) was the first in vivo radiotracer used to image Aβ in the brains of patients with AD and control subjects (
12). PiB is a thioflavin analogue that binds with low nanomolar affinity to aggregated fibrillar deposits of the Aβ peptide, is detectable by PET, and clears rapidly from normal brain tissue (
12). At low concentrations used in PET imaging, PiB selectively binds to fibrillary Aβ deposits in postmortem brain tissue. Moreover, in cortical areas, PiB retention was found to be inversely correlated with regional glucose utilization, determined using FDG (
14). A more recent report examining the relationship of antemortem
11C-PiB binding to postmortem estimate of plaques and tangles found general agreement between regional measures of Aβ, but not tangles, and in vivo PiB retention in the anterior and posterior cingulate gyri and the precuneus (
15).
PiB retention values help separate patients diagnosed with AD and mild cognitive impairment from healthy control subjects cross-sectionally. Further, longitudinal studies indicate that PiB-positive subjects with mild cognitive impairment at baseline are significantly more likely to convert to AD status than PiB-negative subjects (
16). In addition, Morris et al. (
17) have reported that elevated PiB binding in cognitively normal older adults predicts the development of symptoms of dementia. Newer
18F radiotracers that bind to Aβ have been developed over time and include florbetapir, florbetaben, and flutemetamol (
18). They have been approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency for use in the clinical assessment of cognitive disorders in order to exclude AD. They are comparable to one another and PiB in important respects and have the added advantage of being labeled with
18F, which, because of a longer half-life than
11C, facilitates imaging in clinical settings (
18,
19).
[
18F]FDDNP is a small molecule that binds to Aβ and tau (
13,
20,
21). It is therefore less specific than the aforementioned radiotracers that bind exclusively to amyloid, although it more completely images the neuropathological changes observed in AD. Using [
18F]FDDNP in clinical studies, Shoghi-Jadid et al. (
20) showed greater radiotracer retention in several brain regions in the mild AD group when compared with control subjects. In the AD group, retention was greater in the hippocampus, the amygdala, and the entorhinal cortex, where retention was 30% higher than in the pons, which served as the reference region. Like PiB, [
18F]FDDNP helps separate patients with AD from control subjects and also tracks conversion from normal cognitive status to mild cognitive impairment, and from mild cognitive impairment to clinical dementia (
13). In addition, [
18F]FDDNP imaging may help in differentiating different forms of tauopathies (
22,
23). Amyloid and tau imaging done concurrently may help determine the relative contributions of both pathophysiological mechanisms to AD and assess the impact of therapies based on antiamyloid and antitau compounds.
Selective tau imaging.
Because a definitive diagnosis of AD requires neuropathological evidence of both Aβ and tau, precise in vivo tau imaging will greatly advance the field, both from the diagnostic and the therapeutic perspectives. The ideal PET tau tracer would have the following characteristics: high affinity and selective binding to paired helical filaments and phosphorylated tau over Aβ; high blood-brain barrier permeability; and low binding to non-tau proteins in the brain (
24,
25). Unlike the widespread neocortical distribution of amyloid tracers in the neocortex, tau tracers are retained more notably in the inferior temporal and parietal regions of the brain. While tau deposition is widely believed to be a “downstream” event in the pathogenesis of AD, occurring after neocortical deposition of Aβ, the relationship between the two is complex and could be part of a feedback loop (
26). While Aβ imaging shows a modest relationship between amyloid load and cognitive compromise, PET imaging demonstrates a closer relationship between tau pathology and the clinical severity of the dementia (
24,
25). Optimally, comprehensive in vivo neuroimaging in AD should include imaging of both Aβ and tau, as they are both required for a definitive diagnosis of AD, and complementary neuroimaging will increase the accuracy of the diagnosis of AD.
Despite these challenges, several first-generation tau radiotracers were developed and used in clinical research studies in well-characterized patients. Several quinoline derivatives were first identified as candidates for tau binding (
24,
25).
18F-linked compounds, including [
18F]THK-5105 and [
18F]THK-5117, and
11C-related agents, such as PBB3, were developed to improve binding PHF tau over Aβ. Interpretation of some of the THK ligand binding is confounded by the fact that monoamine oxidase B is an off-target binding site (
27). Despite limitations, proof-of-concept studies demonstrate that these tracers separate AD patients from healthy control subjects, and tracer retention correlates significantly with the clinical severity of dementia (
28,
29).
Second-generation tau radiotracers have been developed to overcome some of the earlier limitations, including limited sensitivity and selectivity to neurofibrillary tangles. While the future of in vivo tau neuroimaging in AD is promising, it is fair to say that unlike the Aβ-related radiotracers, the tau tracers have not been completely validated by autopsy studies. Imaging-autopsy correlations are necessary to determine whether tau neuroimaging accurately reflects the PHF tau in the brain. The complexity and variety of tau deposits in the brain in different diseases have also posed a challenge for the development of optimal PET (
30). While the growing number of studies investigating tau PET holds promise in AD research and therapy monitoring, more in-depth work is needed to further understand the role of tau in AD and other tauopathies.