It is well documented that depression is a growing public health concern and a major cause of disability worldwide (
1), leading to a significant reduction in quality of life (
2) and impaired psychosocial functioning (
3). In addition to being an important condition in its own right, depression has been associated with an elevated risk of morbidity (
4), cognitive decline (
5), and mortality (
6). However, the pathophysiology of depression is not fully understood. Approximately one-third of patients with depression fail to respond to conventional antidepressant therapies, and less than 40% obtain remission after initial treatment (
7). Furthermore, there have been no clinically reliable predictors of treatment response to existing antidepressant treatment regimens (
8).
Following the extensive search for biomarkers linked to depression, there has been emerging interest in the role of immune system disturbances, in particular systemic inflammation, in depression etiology. This view is supported by findings on shared genetic variants between the immune system and depression (
9). In addition, proinflammatory systemic cytokines have been found to be capable of affecting depression-related endocrine functioning and neurotransmitter metabolism by traversing the blood-brain barrier or inducing activation of afferent fibers of the vagus nerve (
10,
11), including the central synthesis and reuptake of amine transmitters (
12,
13). To date, however, the collective evidence from population-based cohort studies and clinical trials on the association between systemic inflammation and depression has been inconsistent (
14–
18). Since depression is a multifaceted mental disorder with varying types of symptom expressions (
19), this discordance may be ascribed to symptom-specific effects of inflammation that are lost when a single aggregate measure of depression is used (
20). According to the two most widely used classificatory diagnostic systems of mental disorders—ICD-11 (
21) and DSM-5 (
22)—depression can be broadly classified into emotional (e.g., depressed mood, anhedonia), cognitive (e.g., difficulties concentrating), and physical (e.g., sleep problems, fatigue, changes in appetite) symptoms. Different symptoms may have distinct underlying etiological pathways, but few studies to date have examined the associations between systemic inflammation and individual symptoms of depression (
3,
23–
27). Further limitations in this field of research include the reliance on small sample sizes, insufficient control for potential confounding factors, and a lack of evaluation of temporality and consistency of potential symptom-specific associations across different subgroups and inflammatory markers.
In this multicohort study of 15 population-based cohorts comprising up to 56,351 individuals, we sought to address these limitations by exploring the cross-sectional and longitudinal associations of two systemic inflammatory markers—C-reactive protein (CRP) and interleukin-6 (IL-6)—with 24 individual symptoms of depression, including physical, emotional, and cognitive symptom domains, and biased perceptions of self. To evaluate the robustness of evidence for or against an association with individual symptoms of depression, we explored these associations in subgroups of men and women, subgroups of younger and older adults, among individuals with depression, and after excluding those with high levels of inflammation or chronic illnesses.
RESULTS
The pooled data from 15 cohort studies comprised 56,351 participants with a mean age of 57.8 years (SD=12.0). Of these, 27,125 (48.5%) were men and 29,226 (51.5%) women. The geometric mean was 0.89 mg/L for CRP (95% CI=0.85, 0.94) and 0.74 pg/mL for IL-6 (95% CI=0.70, 0.78). The number of cohorts included in the symptom-specific meta-analyses ranged from 2 to 14, depending on the depressive symptom. Across all cohorts, the weighted mean prevalence of depressive symptoms varied from 1.1% (suicidal ideation) to 21.5% (sleep problems) (see
Table 1). The mean prevalence for overall elevated symptoms of depression was 14.0% (see Table S1 in the
online supplement).
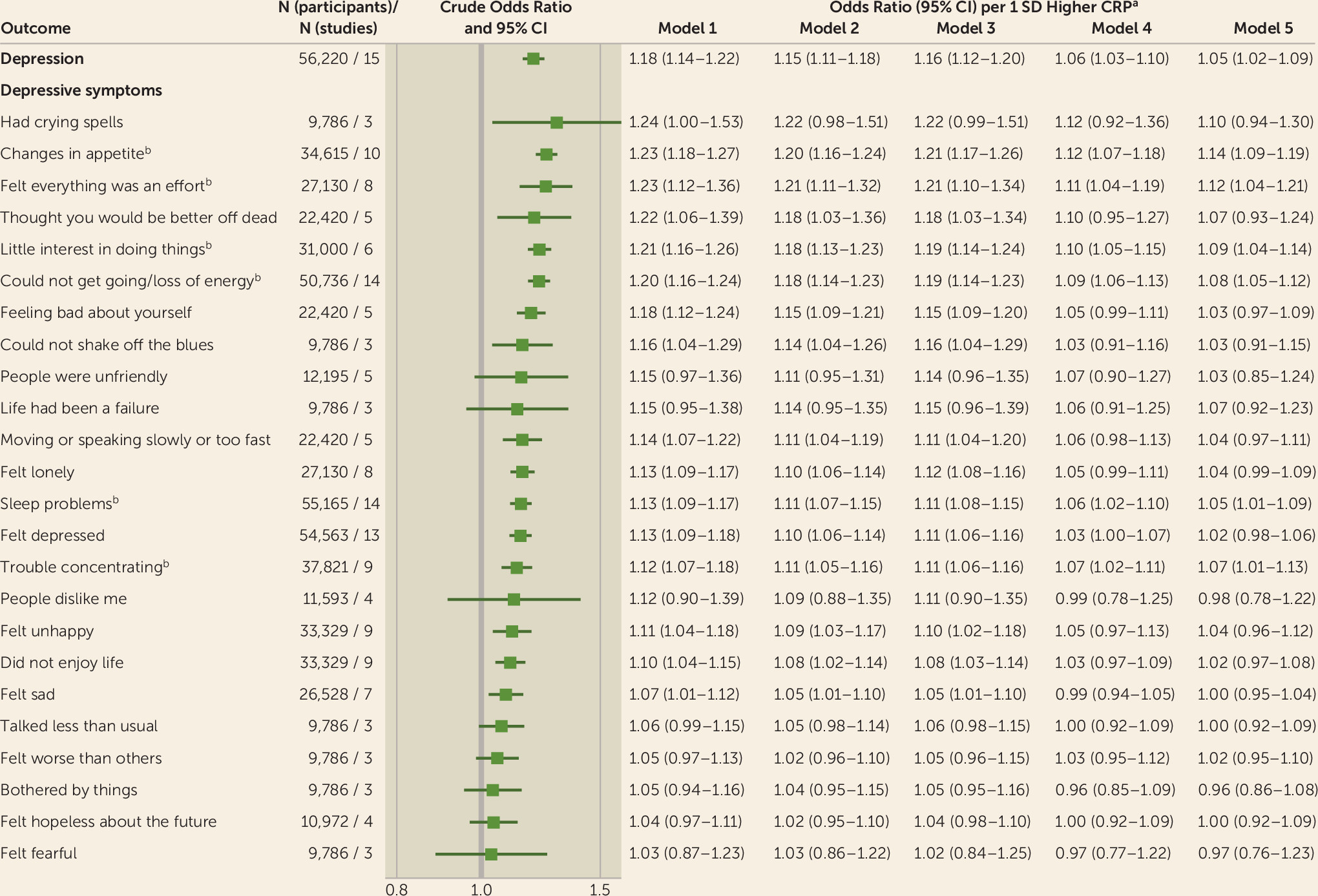
Figure 1 shows the pooled effect estimates of the cross-sectional associations between CRP, depression status, and 24 individual depressive symptoms. In analyses adjusted for age and sex, the odds ratio per one-standard-deviation-higher CRP level for depression was 1.18 (95% CI=1.14, 1.22), but it was attenuated to 1.05 (95% CI=1.02, 1.09) after additional adjustment for sociodemographic, illness-related, and behavioral factors. In symptom-specific analyses adjusted for age and sex, higher CRP concentrations were associated with increased odds of reporting six of seven physical symptoms, two of three cognitive symptoms, five of nine emotional symptoms, two of four biased self-perceptions, and one of one self-harm-related symptom. After further adjustment for socioeconomic, chronic illness–related, and behavioral risk factors, CRP remained robustly associated with four physical symptoms (changes in appetite, felt everything was an effort, could not get going or loss of energy, sleep problems), two cognitive symptoms (trouble concentrating, little interest in doing things/unmotivated), and one emotional symptom (felt depressed). A similar pattern of associations emerged in the sensitivity analysis excluding participants with CRP concentrations ≥10 mg/L, although the association with “felt everything was an effort” was weakened after additional adjustment for behavioral factors (see Figure S6 in the
online supplement). In domain-specific analyses (basic model), CRP was most strongly associated with physical and cognitive symptoms and least associated with emotional symptoms (see Figure S9 in the
online supplement).
Table 2 depicts the pooled effect estimates from cross-sectional analyses for IL-6 and depression status, as well as the individual symptoms of depression that were robustly associated with CRP in our main analysis. Higher IL-6 levels were associated with an increased risk of depression (age- and sex-adjusted odds ratio=1.22, 95% CI=1.09, 1.38), although statistical significance at conventional levels was lost after adjustment for all available covariates (odds ratio=1.08; 95% CI=0.95, 1.23). In symptom-specific analyses (basic model), higher IL-6 levels were associated with increased odds of experiencing five of the seven symptoms previously identified to be robustly associated with CRP in our main analysis (no association was observed with the symptom “felt depressed,” and no data were available to test the symptom “little interest in doing things”). In multivariable-adjusted analyses, point estimates for the association between IL-6 and these five symptoms (odds ratios ranged from 1.09 to 1.31) were similar to or higher than the corresponding point estimates observed in the larger analysis of CRP (odds ratios ranged from 1.05 to 1.14).
To examine generalizability, we assessed heterogeneity (I
2 and τ
2) in study-specific estimates for symptoms that were consistently associated with systemic inflammation in our main analyses (
Table 1). Heterogeneity in study-specific estimates was small or moderate for “changes in appetite” (I
2=0%, τ
2=0.0003), “could not get going/loss of energy” (I
2=21%, τ
2=0.0023), “trouble concentrating” (I
2=22%, τ
2=0.0031), and “little interest in doing things/unmotivated” (I
2=47%, τ
2=0.0010), but high for “sleep problems” (I
2=67%, τ
2=0.0031) and “felt everything was an effort” (I
2=79%, τ
2=0.0142). Of the 14 studies with data on “sleep problems,” 13 supported excess risk (odds ratios >1), and one study favored a protective effect (odds ratio <1). For the symptom “felt everything was an effort,” seven of eight studies supported excess risk in relation to higher CRP concentrations, although the magnitude of the effect estimates varied between cohorts.
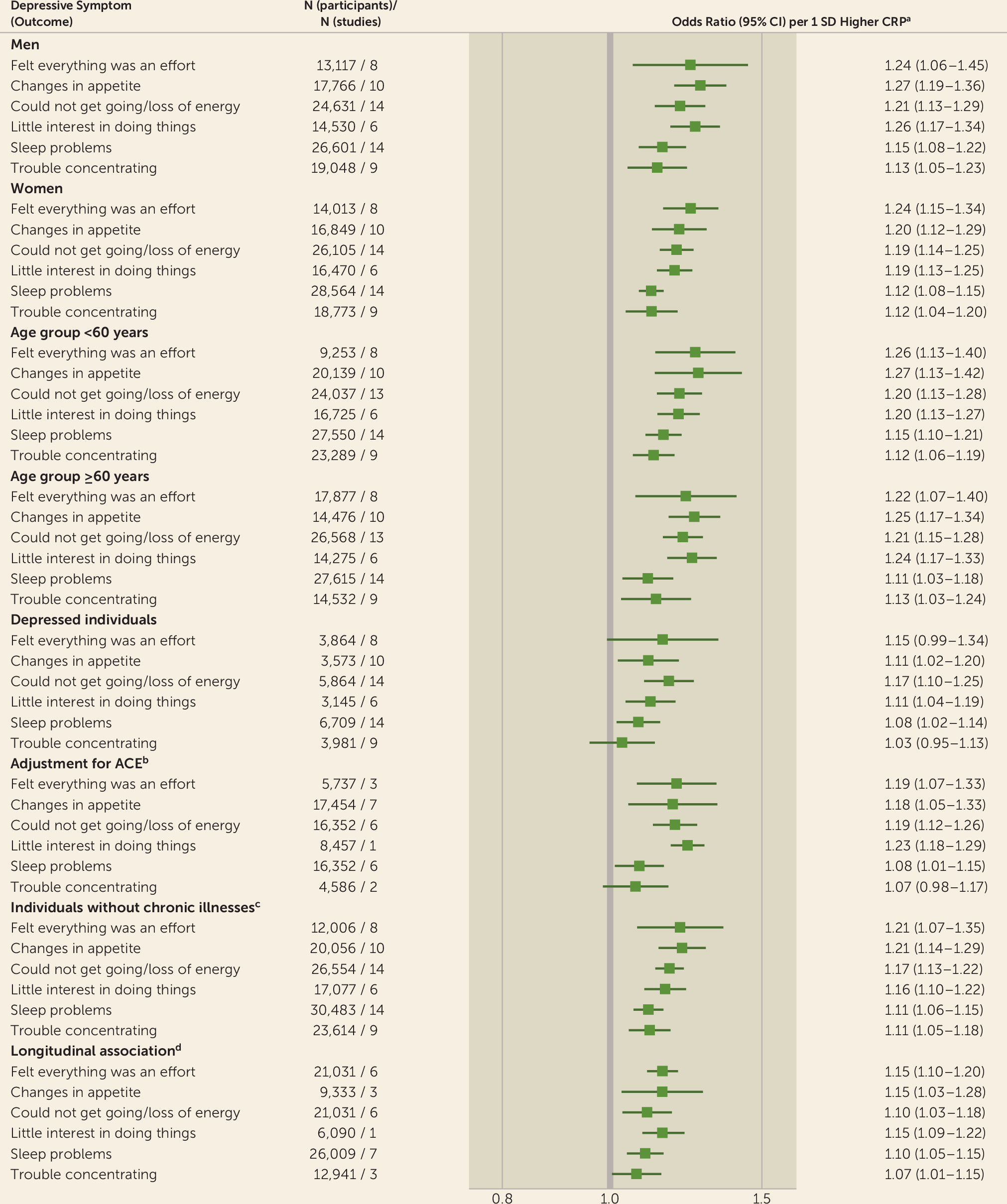
Overlapping point estimates and accompanying 95% confidence intervals in analyses stratified by age, sex, and timing of exposure and outcome measurement at baseline suggest that the inflammation–depressive symptom associations varied little between subgroups (
Figure 2; see also Table S7 in the
online supplement). In depressed individuals, higher levels of CRP were significantly associated with increased odds of reporting “changes in appetite,” “could not get going/loss of energy levels,” “little interest in doing things/unmotivated,” and “sleep problems.” The effect estimate for “felt everything was an effort” was of similar magnitude but with wider 95% confidence intervals. These five robust cross-sectional associations were replicated in analyses excluding chronically ill individuals and in longitudinal analyses (
Figure 2). Additionally, the previously identified longitudinal associations largely remained after multivariable adjustments (see Figure S8 in the
online supplement). In contrast, the association with “trouble concentrating” was attenuated after adjustment for adverse childhood experiences, and no association with this particular symptom was observed in the age- and sex-adjusted analyses among depressed individuals (
Figure 2).
The overall evidence on the symptom-specific associations with systemic inflammation is summarized in
Table 1. In terms of the effect size, robustness to multivariable adjustments, evidence on temporality, consistency across inflammatory markers, and consistency of associations across cohorts and subgroups, strong evidence for an association with systemic inflammation was obtained for three physical symptoms (changes in appetite: age- and sex-adjusted odds ratio=1.23, 95% CI=1.18, 1.27; felt everything was an effort: odds ratio=1.23, 95% CI=1.12, 1.36; could not get going/loss of energy: odds ratio=1.20, 95% CI=1.16, 1.24). Moderate evidence was found for one further physical symptom (sleep problems: odds ratio=1.13, 95% CI=1.09, 1.17) and one cognitive symptom (little interest in doing things/unmotivated: odds ratio=1.21, 95% CI=1.16, 1.26). Moreover, the overall evidence was strongly against an association with four emotional symptoms (bothered by things: odds ratio=1.05, 95% CI=0.94, 1.16; felt hopeless about the future: odds ratio=1.04, 95% CI=0.97, 1.11; felt fearful: odds ratio=1.03, 95% CI=0.87, 1.23; life had been a failure: odds ratio=1.15, 95% CI=0.95, 1.38), while for the remaining 14 symptoms the evidence was mixed (see also Figure S10 in the
online supplement).
Post Hoc Analysis
Further analyses confirmed that individuals with both elevated levels of CRP and high levels of the five inflammation-related symptoms represented a distinct subpopulation. The number of individuals with high CRP concentrations and high symptom levels was 1.3 times higher than expected (O/E=1.31, χ
2=472.5, df=3, N=56,351, p<0.001). They differed from other participants in terms of sociodemographic, behavioral, and illness-related profiles, the most observable differences being their lower educational qualifications, lower physical activity levels, higher BMI, and higher prevalence of diabetes, coronary heart disease, stroke, and cancer, but lower alcohol consumption (all p<0.001) (see Table S5 in the
online supplement). Among depressed people, a similar pattern emerged: elevated levels of CRP and high inflammation-related symptom levels also appeared to denote a specific subpopulation (O/E=1.11, χ
2=108.1, df=3, N=6,814, p<0.001) with a specific risk factor profile (see Table S6 in the
online supplement).
DISCUSSION
Our findings from up to 15 cohort studies comprising 56,351 adults across multiple countries suggest that systemic inflammation is robustly associated with a distinct set of symptoms, both physical (changes in appetite, felt everything was an effort, could not get going/loss of energy, sleep problems) and cognitive (little interest in doing things/unmotivated). These associations were evident across subgroups and two inflammatory biomarkers, in analyses excluding participants with CRP values ≥10 mg/L, after adjustment for socioeconomic, behavioral, and disease-related factors, and in analyses additionally controlling for adverse childhood experiences. In contrast, we found strong evidence against an association with a number of exclusively emotional symptoms, including fearfulness, feeling bothered by things, hopelessness about the future, and feeling life had been a failure.
These results confirm the findings of previous cross-sectional studies showing differential associations between systemic inflammation and changes in appetite (
3,
23,
27), lower energy levels (
3,
23,
24), and sleep problems (
3,
23,
24). Furthermore, in a recent report using NHANES data on 15,071 U.S. adults (
23), a weak association was reported between CRP and little interest in doing things.
In individuals with depression, inflammation was associated with “changes in appetite,” “could not get going/loss of energy,” “little interest in doing things/unmotivated,” and “sleep problems.” The effect estimate for “felt everything was an effort” was comparable to or higher than those for the other four symptoms, but less precisely estimated. In contrast, although we found a robust association with the symptom “difficulties concentrating” in our main analysis, this relationship was absent in the subgroup of depressed individuals. Thus, our findings support the notion that in depressed individuals, systemic inflammation is primarily associated with physical symptoms and the anhedonia-related symptom “little interest in doing things.” If causality is confirmed in future studies, the identified symptom profile could be used to define a subpopulation of depressed people most likely to benefit from anti-inflammatory therapies.
Findings from our longitudinal analyses are partially consistent with two previously published smaller-scale studies. For example, a longitudinal investigation of 2,731 children found that higher IL-6 levels, but not CRP levels, were linked to an increased risk of experiencing concentration difficulties at follow-up, in addition to diurnal mood variation, sleep problems, and fatigue (
26). In an analysis of 2,872 Dutch adults, a higher basal inflammation index, as indicated by increased mean levels of CRP, IL-6, and tumor necrosis factor–alpha, was associated with subsequent physical symptoms (e.g., changes in appetite, sleep problems, and lower energy levels) (
39). However, the latter findings should be interpreted with caution, given the study’s relatively small sample size, an overrepresentation of women, and the limited set of covariates controlled for in the analyses. Moreover, collapsing biologically distinct inflammatory markers into a single mean index of basal inflammation may mask biomarker-specific effects on depressive symptomatology. Sources of heterogeneity in previous studies include differences in study design, sample size, methodology, sample characteristics, and varying statistical adjustments used to control for the influence of potential confounding factors. In the present study, we attempted to move beyond these differences by harmonizing the data from 15 population-based cohort studies, employing a rigorous statistical approach, and adjusting for the effects of a wide range of covariates.
Our results lend support to the sickness behavior theory (
40), which posits that peripherally localized inflammatory activity can initiate a cascade of initially adaptive depressive-like symptoms in a subset of people, collectively known as sickness behavior. These include a lack of energy (lethargy), changes in appetite, sleepiness, reduced social exploration, and, at times, confusion. Sickness behavior is also characterized by depressed mood and increased sensitivity to pain (hyperalgesia). In the present study, evidence on the association between inflammation and depressed mood was mixed, and no data were available on hyperalgesia. In addition, we found strong evidence against an association between inflammation and four exclusively emotional symptoms: “bothered by things,” “felt hopeless about the future,” “felt fearful,” and “life had been a failure,” most of which have previously been classified as non–sickness behaviors (
39).
Our multivariable-adjusted findings highlight the potential contributions of lifestyle factors to the CRP-depression association because adjustment for lifestyle covariates led to substantial attenuation, an observation that accords with findings from a recent large-scale case-control study (
41). The latter investigation found that the association between CRP and overall depression was strongly attenuated but remained statistically significant at conventional levels after controlling for the effects of age, sex, early-life trauma, self-reported health status, alcohol intake, smoking, and BMI. Of these, smoking and BMI appeared to be the most influential factors. Similarly, BMI exerted the greatest attenuating effect in our analyses. This suggests that a high BMI may contribute to both inflammation and depressive symptoms, lie on the causal pathway between inflammation and depressive symptoms, or both (
3,
18,
24,
27,
42). IL-6 and CRP are synthesized in response to adipocytes within adipose tissue (
43), and higher levels of body fat, in particular visceral fat, are related to metabolic inflammation and depression (
44,
45). Moreover, we found that adjustment for BMI particularly reduced the strength of the relationships between inflammation and physical or energy-conserving symptoms, including “changes in appetite,” “lower energy levels,” and “sleep problems”—a finding supported elsewhere (
46). The precise drivers and mechanisms by which increased levels of circulating inflammatory markers exert an influence on depressive symptoms remain unclear. Further research is needed to dissect the interplay between metabolic conditions, inflammation, and individual symptoms of depression and to examine whether other sources, such as genetic susceptibility to systemic inflammation or stress-related responses of the body, may underlie the inflammation–depressive symptom association (
47).
Our results may have important implications for future research by suggesting a more targeted, symptom-focused approach to exploring the link between systemic inflammation and depression, particularly in anti-inflammatory drug trials. Current evidence from early-stage clinical trials on the effect of anti-inflammatory drug therapies on depression is sparse, and interpretation of findings is hampered by methodological heterogeneity across studies (
48,
49). First results indicate modest antidepressant effects of cytokine inhibitors and celecoxib add-on therapy to conventional antidepressants in depressed patients with prior elevated levels of systemic inflammation (
50). However, clinical studies on possible symptom-specific effects of anti-inflammatory therapies are still missing.
The present study has a number of strengths, including its large sample size, which offers higher statistical precision than extant studies; the use of more than one inflammatory marker to assess systemic inflammation; ascertainment of the robustness of associations in multivariable-adjusted analyses; confirmation of the generalizability of the findings across multiple cohorts and subgroups; and the use of longitudinal data to assess temporality. However, our results need to be interpreted in light of some limitations. Causal inference is limited by the use of observational data. Our exposure variable, systemic inflammation, was measured only once at baseline; repeated measurement of inflammatory markers would have enabled us to better capture the time-varying nature of inflammation. Measurement error due to diurnal changes in IL-6, fasting status, and analytical methods may have biased the estimates of our secondary exposure. The primary analysis was based on CRP—a largely stable biomarker which is unaffected by diurnal variations (
51). However, IL-6 is less stable and fluctuates throughout the day. In this study, measurement of IL-6 was based on serum blood samples collected in the morning after overnight fasting in all cohorts. Hence, confounding due to variability in the time of blood collection, analytical method, and fasting status is likely to be limited in our sample. A further limitation is that the assessment of depressive symptoms was based on self-report rather than clinical interviews, which are considered to be the gold-standard method in psychiatric research. In addition, although we included a wide range of covariates, we were not able to control for the influence of anti-inflammatory, antidepressant, or anticoagulation drugs, which may have modified the association between systemic inflammation and depressive symptoms.
In summary, we found that circulating inflammatory markers were robustly associated with a defined set of physical and cognitive symptoms. There was equally strong evidence against an association with exclusively emotional symptoms characterized by fearfulness and negative feelings about life and the future. These findings are largely consistent with the sickness behavior theory of depression. The scientifically reliable identification of symptom-specific associations with inflammation is valuable, as it demonstrates a differential rather than a generalized effect of inflammation on depression. Hence, our findings may pave the way toward a new inflammatory depression phenotype and can guide systematic efforts to develop novel inflammation-targeted treatments. Patient recruitment to future anti-inflammatory drug trials should be based on symptom profiles characterized by the inflammation-related depressive symptoms observed in this study.