Major depressive disorder (MDD) is one of the leading causes of disability worldwide (
1), with a total yearly economic burden in the United States alone estimated at over $200 billion (
2). MDD is associated with chronic physical symptoms (
3), increased risk of suicide (
4), and dementia (
5). While many adult studies of MDD typically focus on younger and midlife adults, this disorder affects people throughout the lifespan (
6). Late-life depression (LLD), defined as the presence of MDD in individuals older than 55, is associated with cerebrovascular disease (
7) and Alzheimer’s disease (
8). However, LLD can refer to people older than 55 with a history of depression (early onset) and those older than 55 who develop depression for the first time (late onset). Some have argued that LLD with late onset is a “different” disorder that should be studied separately from MDD (
8,
9). An assessment of the brain circuitry in younger patients with MDD, older patients with early-onset MDD, and older patients with late-onset MDD could help to clarify how the same depressive syndrome in these three groups may be associated with shared as well as unique neuropathology.
Considerable efforts have been dedicated to uncovering the neural mechanisms underlying MDD in structural neuroimaging studies. Meta-analyses of brain structure in MDD suggest that structural abnormalities are present in the prefrontal cortex and medial temporal lobe regions such as the hippocampus, parahippocampal gyrus, and amygdala (
10–
12). A large-scale analysis (N=2,148 MDD patients) of a data set from the Enhancing Neuroimaging Genetics Through Meta-Analysis reported cortical thinning in the orbitofrontal cortex (OFC), anterior and posterior cingulate, insula, and temporal lobes (
13). Further, smaller volumes of the OFC (and gyrus rectus), frontal lobe, basal ganglia, thalamus, and hippocampus have been reported as loci of structural differences in MDD (
14). Meta-analytic evidence suggests that both MDD and LLD affect the hippocampus, amygdala, and ventral anterior cingulate, while LLD uniquely affects the visual cortex (
11). Mechanistic theories propose that LLD (and late-onset LLD in particular) is characterized by fronto-executive impairments due to cerebrovascular disease (
15).
An important challenge regarding structural neuroimaging findings in MDD and LLD is the large degree of heterogeneity (
8,
10) attributable to differences in demographic or clinical characteristics (
16), depression “biotypes” (
17), or study methodology. For instance, greater volume or thickness in several regions affected in MDD (notably cingulate, orbitofrontal, medial, and dorsolateral prefrontal cortices) is associated with remission after antidepressant treatment (
18). In LLD specifically, age at onset may be associated with different etiologies and may produce different results (
19). Going beyond systematic review, activation likelihood estimation (ALE) (
20) is the current gold-standard approach to synthesizing whole-brain neuroimaging findings, yet it did not detect convergence in voxel-based morphometry findings in MDD (
10). Given this heterogeneity in findings, a novel, more sensitive coordinate-based network mapping approach may be needed to characterize the neural circuitry underlying MDD and LLD (
21–
23).
We carried out a systematic review and meta-analysis of the scientific literature to identify specific networks affected in major depression in younger to middle-aged adults with MDD and older adults with LLD. Using ALE, we expected clusters of shared structural differences to emerge in the ventromedial prefrontal cortex and the hippocampus in both MDD and LLD (
10,
11). Using coordinate-based network mapping, we expected large-scale impairments in the frontoparietal control network (
24), the dorsal attention network (
25), and possibly the default mode network (
26) in both MDD and LLD. We also hypothesized impairments in the visual network in LLD but not in MDD (
11). Finally, we expected that different demographic and clinical characteristics, notably age at onset of LLD and use of antidepressants in MDD, would be associated with different findings, in particular greater involvement of the aforementioned networks in late-onset than in early-onset LLD.
RESULTS
A total of 143 articles, reporting on 145 comparisons (128 MDD comparisons [depressed participants, N=6,362; control participants, N=6,953] and 17 LLD comparisons [depressed participants, N=535; control participants, N=468]), were included in the analyses (
Figure 1). Of these, 102 studies were based on VBM results, and the remaining 43 studies used surface-based cortical thickness measures (conducted in FreeSurfer). The mean age was 37.2 years (SD=6.7, range=21–50) in the 128 MDD studies and 68.4 years (SD=6.1, range=55–79) in the 17 LLD studies. Participants in all 145 studies fulfilled the criteria for a DSM or ICD diagnosis of MDD, and all studies had high scores on the modified Newcastle-Ottawa Scale, which assesses the quality of nonrandomized studies. In all LLD studies, the mean score on the Mini-Mental State Examination was >24.
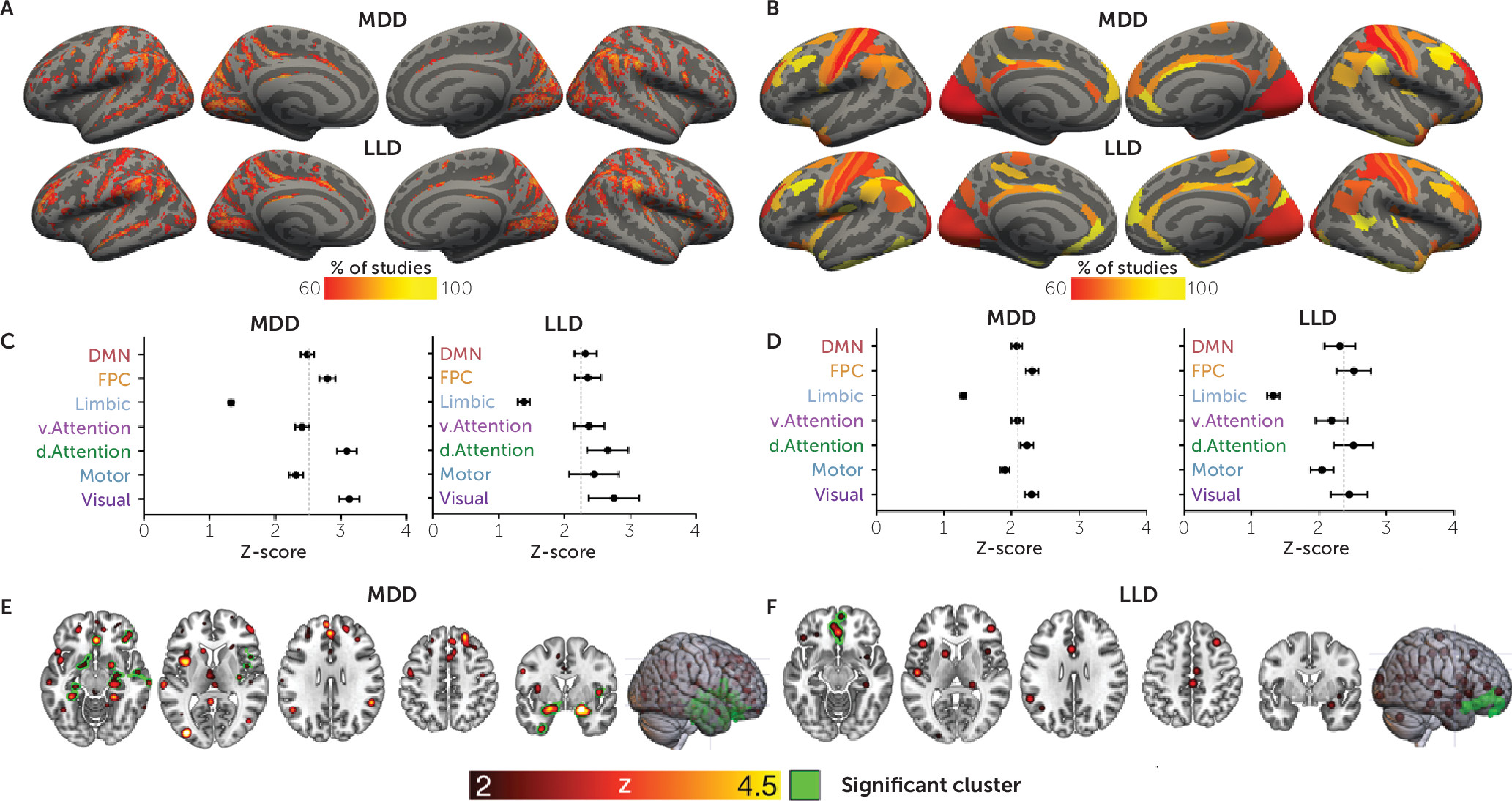
For both MDD and LLD, there were qualitative differences in brain structure in a heterogeneous set of brain areas. In MDD studies, ALE meta-analysis revealed two significant clusters (
Figure 2E; see also Table S2 in the
online supplement). These clusters robustly implicated medial temporal lobe regions bilaterally, including the parahippocampus (Z=5.2, p
FWE<0.001) and ventral anterior cingulate (Z=4.5, p
FWE<0.001, extending to the subgenual anterior cingulate cortex [ACC]), along with right striatal nuclei (putamen and caudate) and the right insular cortex and right inferior frontal gyrus (IFG) (p
FWE<0.01). Using ALE meta-analysis in LLD, we identified a significant cluster in the ACC and medial prefrontal cortex (Z=3.7, p
FWE<0.001) (
Figure 2F; see also Table S2 in the
online supplement).
Coordinate-based network mapping captured large-scale networks implicated in both MDD and LLD. Group mean network maps in MDD and LLD were similar (
Figure 2A,C), reliably implicating the inferior frontal gyrus and parietal-opercular gyri (supramarginal, angular gyrus) along with the middle frontal gyrus, frontal pole, anterior cingulate, and visual cortices in over 60% of the studies (
Table 1).
The three-way ANOVA identified a significant main effect of the Yeo networks (F=42.8, df=3, 430, p<0.001). Post hoc comparisons indicate that across MDD and LLD studies, differences between patients and control subjects were centered on the frontoparietal control, dorsal attention, and visual networks, since those networks showed significantly greater involvement than the limbic network (
Figure 2B,D). Less differentiation was found with the ventral attention and default mode networks. No significant main effects of group were found (F=0.35, df=1, 143, p=0.85), and a small but significant effect of modality was found (F=4.7, df=1, 143, p=0.032). No significant two-way interactions between Yeo networks and group (MDD versus LLD, F=0.4, df=3, 430, p=0.75) or modality (morphometry and resting state) and group (F=1.0, df=1, 143, p=0.32) were found, suggesting that the involvement of the Yeo networks was the same for both MDD and LLD and that these two groups did not show different patterns in the resting-state versus morphometric similarity analyses, respectively. Further, no significant three-way interaction between Yeo networks, modality, and group was found (F=1.2, df=3, 426, p=0.29), suggesting that the pattern of Yeo network differences in the MDD and LLD groups was the same for the morphometric similarity and resting-state connectivity analyses.
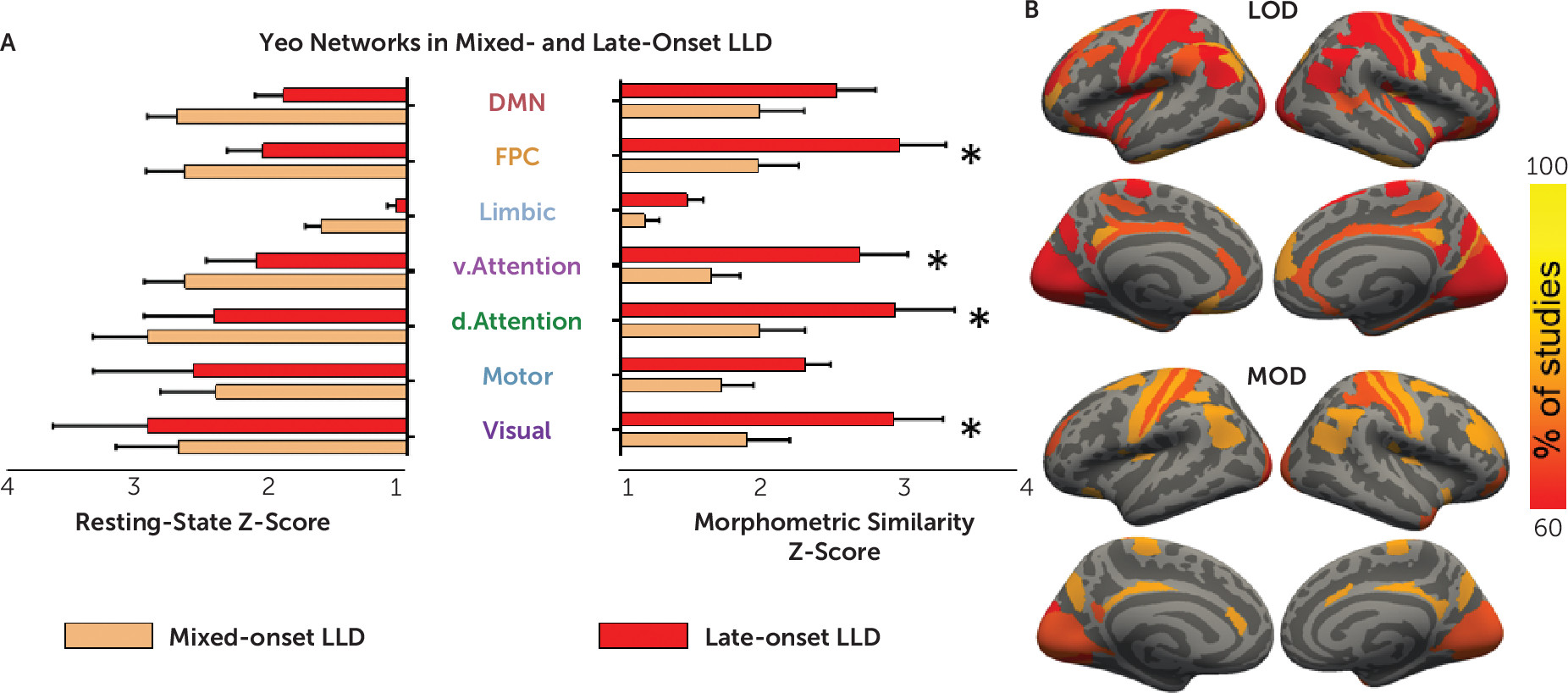
A three-way ANOVA confined to the 17 LLD studies revealed a significant main effect of Yeo networks (F=14.9, df=2.5, 38, p<0.001), replicating results shown in
Figure 2. This three-way ANOVA showed no significant three-way interaction (F=1.2, df=2.7, 40, p=0.33) or Yeo network–by–onset group interaction (F=1.8, df=2.5, 38, p=0.16). The ANOVA revealed a significant interaction of modality with early/mixed-onset versus late-onset group (F=4.9, df=1, 15, p=0.04) (
Figure 3A). In a follow-up two-way ANOVA focusing on the morphometric similarity analysis, frontoparietal, dorsal, and ventral attention and visual networks were significantly more affected by late-onset LLD than early/mixed-onset LLD. Motor, limbic, and default mode networks were implicated at a similar level in both onset groups. The late- and mixed-onset studies did not differ in age, sex, depression severity, or use of antidepressants (data not shown; all p values for one-way ANOVAs >0.05). No main effect of group was found in the three-way ANOVA (F=1.2, df=1, 15, p=0.3) since the differences between the late- and mixed-onset groups were only found using morphometric similarity and not resting-state connectivity.
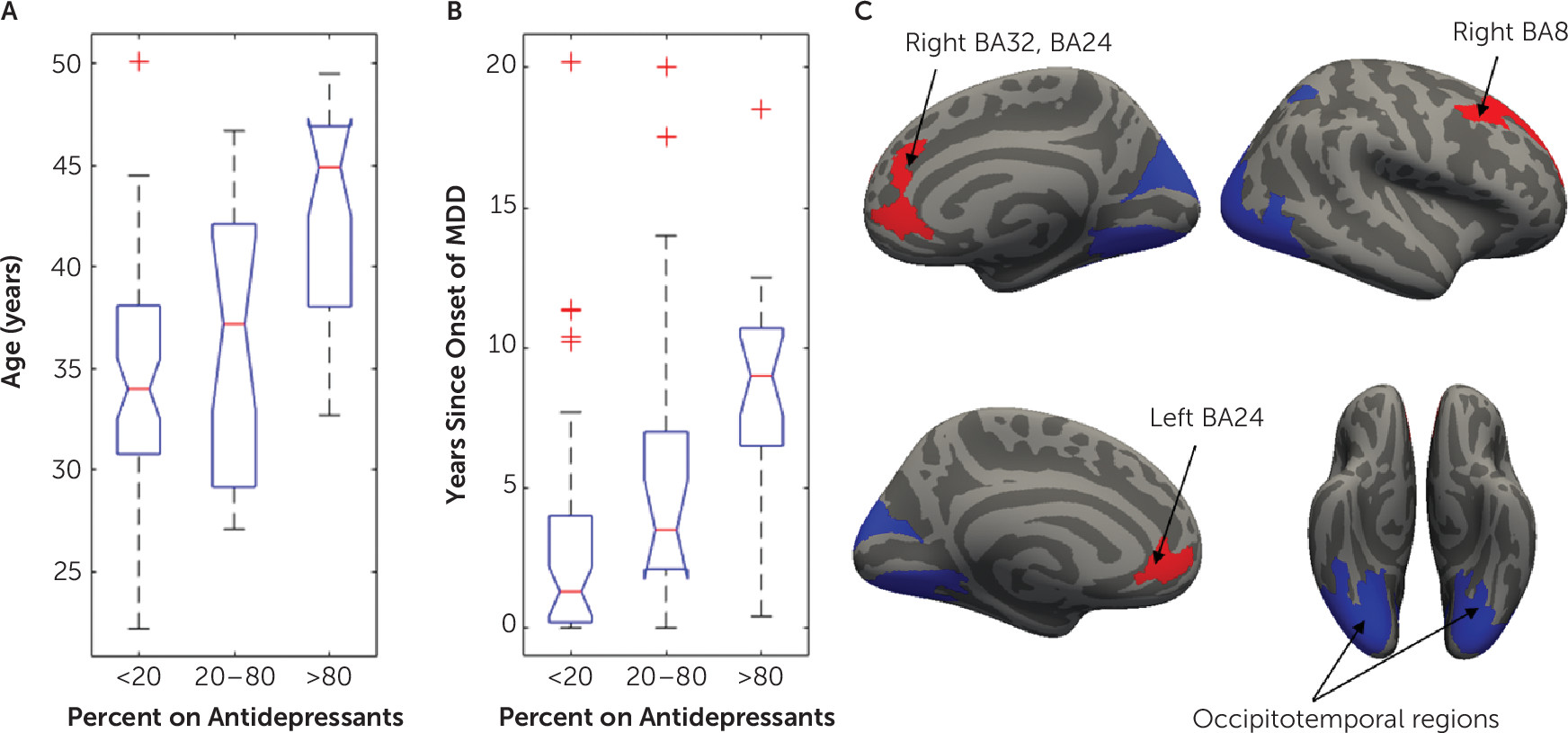
The final analysis investigating the effect of demographic and clinical factors on brain heterogeneity in younger adult MDD included 114 MDD studies, as 14 studies were excluded because of insufficient information on use of antidepressants. In MDD studies, the proportion of patients currently taking an antidepressant had a significant effect on the networks affected by structural abnormalities identified using resting-state network mapping (
Figure 4C). More specifically, MDD studies that included more patients on antidepressants showed greater involvement of networks involving the dorsal and ventral ACC and dorsolateral prefrontal cortex (dlPFC) (t>2.36, df=1, 108, p
FDR<0.05). By contrast, studies that included fewer patients on antidepressants found greater differences in occipitotemporal regions (t<−2.39, df=1, 108, p
FDR<0.05). A sensitivity analysis included 34 first-episode studies and replicated the ACC findings (t>2.1, df=1, 27, uncorrected p<0.05; see section 2.3 in the
online supplement), although it did not survive correction for multiple comparisons (p
FDR>0.05). When repeating the above analyses with morphometric similarity network mapping in MDD studies, no significant effects of sex, use of antidepressant, or depression severity were found in Glasser space (p
FDR>0.05).
Since an effect of the use of antidepressants was found, we assessed the effects of use of antidepressants on age, sex, depression severity, and years since the onset of depressive symptoms in MDD. Age at onset was not available for 34 MDD studies; therefore, we included this variable only for post hoc examination of clinical characteristics of different MDD subgroups. For these follow-up analyses, three groups of MDD studies were formed, according to the proportion of participants taking antidepressants (
Figure 4).
Studies including more patients taking an antidepressant reported a higher mean age (
Figure 4A) (F=21.4, df=2, 111, p<0.001) and a higher number of years since onset of MDD (
Figure 4B) (F=10.6, df=2, 84, p<0.001). Twenty-nine of the 57 studies in which patients were not receiving antidepressants were focused on first-episode depression. In comparison, only five of 57 studies in which patients were receiving antidepressants included patients with first-episode depression. There were no significant associations between the proportion of participants taking antidepressants and depression severity (χ
2=9.5, p=0.05) or sex (F=0.95, df=2, 114, p=0.39).
No significant effects of demographic, clinical, or quality control variables on networks impaired in LLD were found (morphometric similarity or resting-state networks, pFDR>0.05 in Glasser space).