Given the neurodevelopmental origins of autism (
13), the underlying biological mechanisms are likely to act early ontogenetically. The prenatal period is a critical developmental window in which genetic, hormonal, and neuroimmune organizational factors have long-lasting effects on sexual differentiation and pathways implicated in neurodevelopmental conditions such as autism (
14). Different etiological models have been put forward explaining the sex-differential autism likelihood (
15–
17), for example, the female protective effect (
18–
20) and increased steroidogenic activity in prenatal neurodevelopment (
15,
16,
21,
22). Thus, a key question is how proposed explanations translate into neurobiological cascades from genome to neural and behavioral phenotype. Specifically, we need to better understand how sex-differential neural phenotypes are linked to cognition and genomic mechanisms, and whether they can be traced back to the prenatal developmental period and specific pathways. Recent work shows that studying transcriptional patterns that spatially overlap with brain structural changes provides a mechanistic lens into the physiology of neurodevelopmental conditions (“the transcriptional vulnerability model” [
23,
24]). Brain structure is particularly relevant in the developmental biology of neurodevelopmental conditions such as autism (
25,
26) and can be considered an intermediate phenotype between genetics and behavior (
27). Specifically, autism has been associated with spatially selective alterations in neuroanatomy that have been linked to both autistic behavioral features (
28) and genes relevant to these features (
29–
31).
Motivated by the need to address previous shortcomings, such as small sample sizes (especially of females) and lack of independent replication, tests of methodological robustness (
32), and careful accounting for confounding variables (e.g., brain volume [
7]), our aim in this study was to comprehensively establish whether the neuroanatomy in male and female autistic people shows, on average, a shift toward (and beyond) the typical male neuroanatomy, and whether this is associated with autism-associated and typically sex-differential cognition and related gene expression. In line with etiological models and evidence suggesting that typical male neurobiology is associated with a higher likelihood of autism (
14,
16,
19,
21), we derived three hypotheses, using a T
1-weighted structural brain imaging–based sex prediction classifier pretrained in neurotypical males and females to predict male sex: 1) among autistic individuals, females will be less accurately classified than males; 2) among females, autistic individuals will be less accurately classified than neurotypical individuals; and 3) among males, autistic individuals will be more accurately classified than neurotypical individuals. We further examined whether male-shifted neuroanatomy is specific to autism; neurodevelopmentally different across age; associated with specific brain regions and clinical features; and linked to gene expression associated with autism, prenatal development, or sex differences.
To test our hypotheses, we employed a novel deep learning framework leveraging several large-scale neuroimaging data sets with proportionally large numbers of autistic females: the UK Biobank (
33), comprising over 10,000 individuals; the Autism Brain Imaging Data Exchange (ABIDE [
34,
35]); and the EU-AIMS/AIMS-2-TRIALS Longitudinal European Autism Project (LEAP [
36,
37], the largest European multicenter initiative aimed at identifying biomarkers in autism). Based on T
1-weighted structural brain images, we first established a sex prediction classifier with high accuracy in the UK Biobank, next transferred and validated it in ABIDE, and then tested it in LEAP to assess sex classification accuracies in autistic males and females. To establish the specificity of our results to autism, we then applied the sex prediction model to an independent sample of individuals with attention deficit hyperactivity disorder (ADHD)—another male-biased neurodevelopmental condition.
Discussion
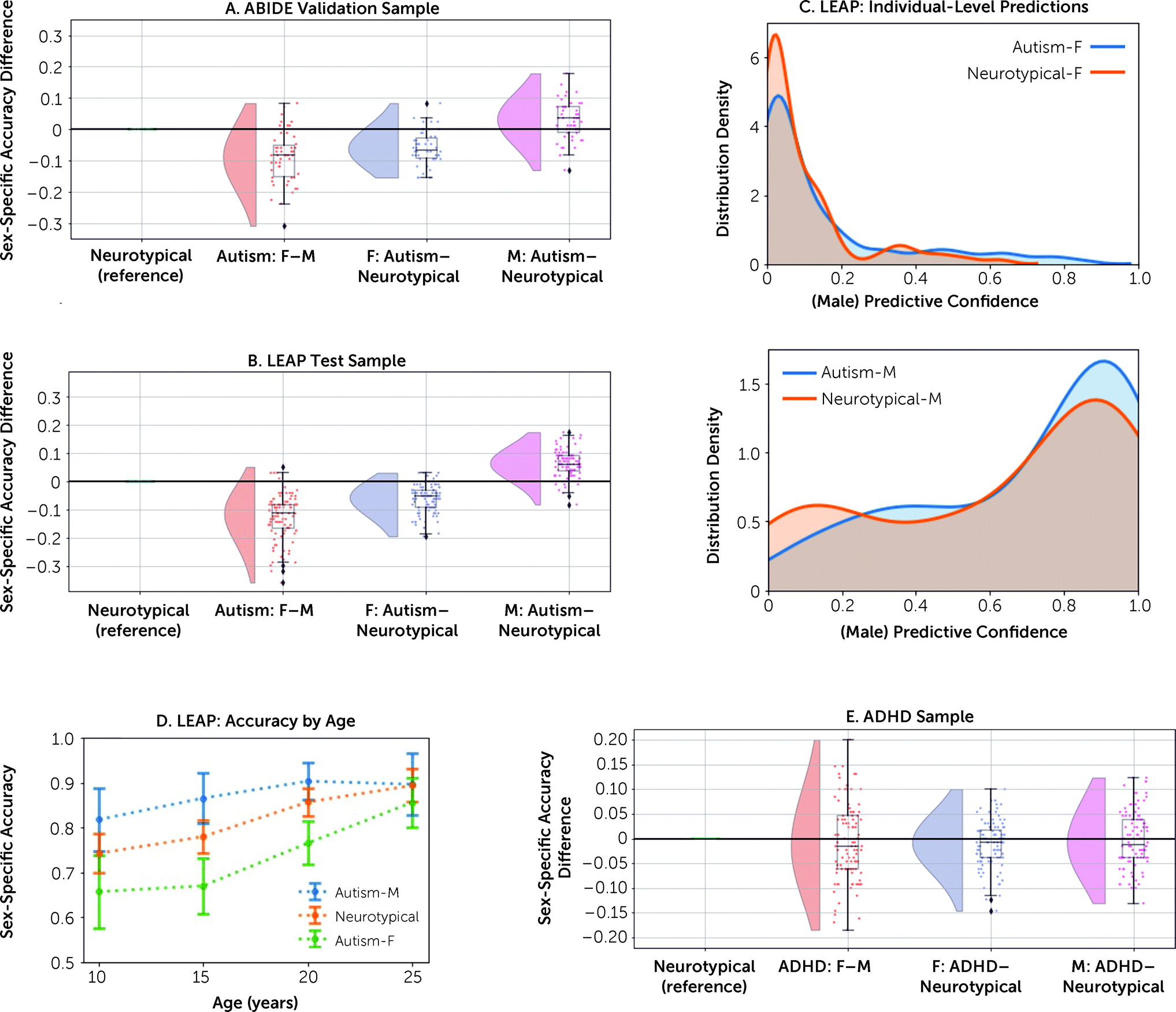
Our study demonstrates the overlap of neuroanatomical features characteristic of neurotypical males with those of autistic individuals. This pattern was specific to autism and was not observed in ADHD, pointing to possible different underlying biology in different neurodevelopmental conditions, despite both having a male-predominant prevalence. Overall, autistic females constituted the more critical test case for our hypotheses, likely because masculinizing sex-differentiation effects are less likely to reach a ceiling in females. Consistent with this, we observed an association between greater shifts toward neuroanatomical maleness and cognitive functional difficulties in social sensitivity and emotional face processing in autistic females. Genes relevant to brain regions predictive of male sex in autistic females were highly similar to those differentiating neurotypical males from neurotypical females, primarily comprising prenatal cell types (excitatory neurons) and upregulated autism-associated genes. The findings provide key insights into potential neurobiological and genomic underpinnings associated with male-biased autism prevalence.
We emphasize that the majority of autistic females were correctly classified by sex, and we cannot derive individual-level predictions of autism given the overlapping distributions across neurotypical and autistic individuals. This highlights the large heterogeneity inherent to autism and the importance of identifying biologically meaningful subgroups (
65,
66). Among these, at least one subgroup is characterized by multivariate features in brain structure that are more similar to those of neurotypical males. These multivariate results of male-shifted whole-brain patterns add to previous mass-univariate neuroimaging studies that identify shifts toward the neurotypical male profile in autistic females across both brain structure (
6,
8,
67) and function (
10,
11,
32). A male-like profile in autistic individuals has previously been reported in specific aspects of cognitive style (
68,
69). Our study extends this observation to multivariate, male-shifted characteristics in brain structure. Importantly, the findings are in line with the notion that there is no strict sexual dimorphism in human neuroanatomy (
70,
71), but brains exhibit a “multimorphic” mosaic of male-like and female-like features (
72,
73) that can reliably distinguish males from females with above-chance to high accuracy (
72,
74,
75). This means that not all autistic individuals have an “extreme male brain,” but multivariate patterns characteristic of neurotypical males are on average more common in a subset of autistic individuals who are overall shifted toward the male neurophenotype. We previously reported such functional brain mosaicism in autistic males who exhibited both shifts toward male- and female-like network connectivity depending on the neural circuit involved (
12). We thus emphasize the need for future studies to also examine the shifts toward a female-like neurophenotype in autism (
76).
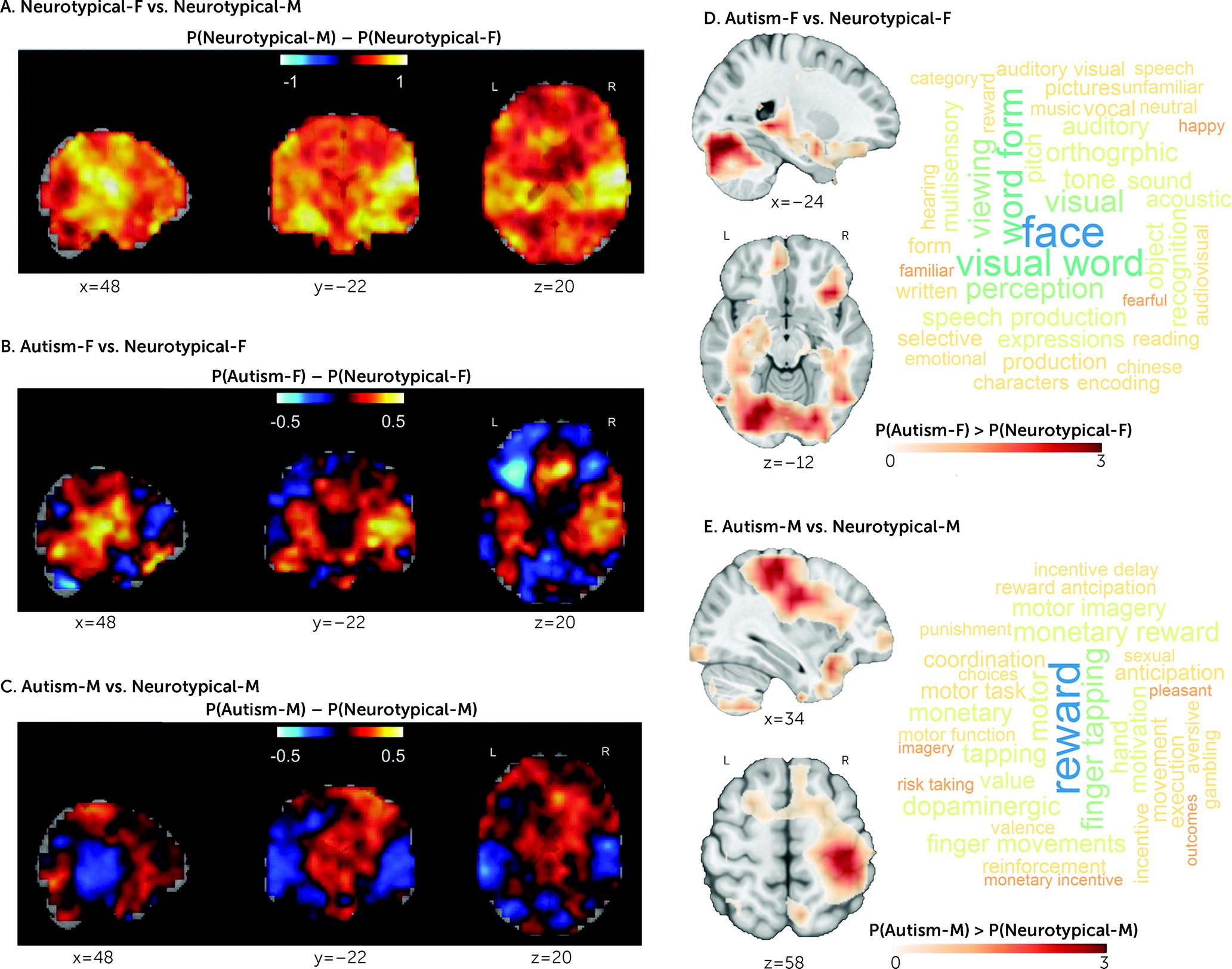
One striking finding is the similarity of neuroanatomical and associated transcriptional patterns between male-shifted regions in autistic females and regions differentiating neurotypical males from neurotypical females. Regions most strongly differentiating neurotypical males from neurotypical females overlapped with well-established sex-differential regions in posterior auditory and visual regions and hippocampus (
77,
78). These visual, face, and language processing areas were also among those that least accurately predicted female sex in autistic females and have previously been associated with male-shifted patterns in autistic females (
6,
32,
79) as well as with atypical brain structure in autism (
80,
81). Higher predicted maleness was also associated with cognitive difficulties in autistic females such as poorer mentalizing and emotional face processing, pointing to the clinical relevance of a male-shifted neurophenotype in autistic females (
16,
68). Furthermore, both sex-differential and the cell-type-specific expression patterns overlapping with male-shifted regions in autistic females and with regions differentiating neurotypical males from neurotypical females were highly similar to each other. These multilevel findings jointly suggest that cellular mechanisms mediating neurotypical brain sexual differentiation likely play a role in the etiology of autism, especially in females.
In autistic males, on the other hand, the male-shifted regions were particularly relevant to reward processing and motor functions. The former has been implicated in recent work showing striatal hypoactivation during reward processing in autism (
82) and male-specific atypicality in reward learning in an animal model of autism (
83). Also, somatomotor networks have frequently been found to be atypical in autism (
55,
84–
86), and bilateral volumetric increases in somatosensory, motor, and premotor cortex have been associated with fetal testosterone exposure (
87).
Particularly, the influence of prenatal masculinization via androgens has been suggested to play a mechanistic role in the etiology of autism (
15,
16,
61,
76,
88,
89). Other steroid hormones, including estrogens, have also been found to be associated with autism-related outcomes, when cohorts were restricted to males (
21,
22,
90). Also, neuroimaging research shows that gray matter volumes in posterior auditory regions (exhibiting male shifts in autistic females here) and in somatomotor areas (exhibiting male shifts in autistic males here) are associated with increased androgen sensitivity (
87,
91).
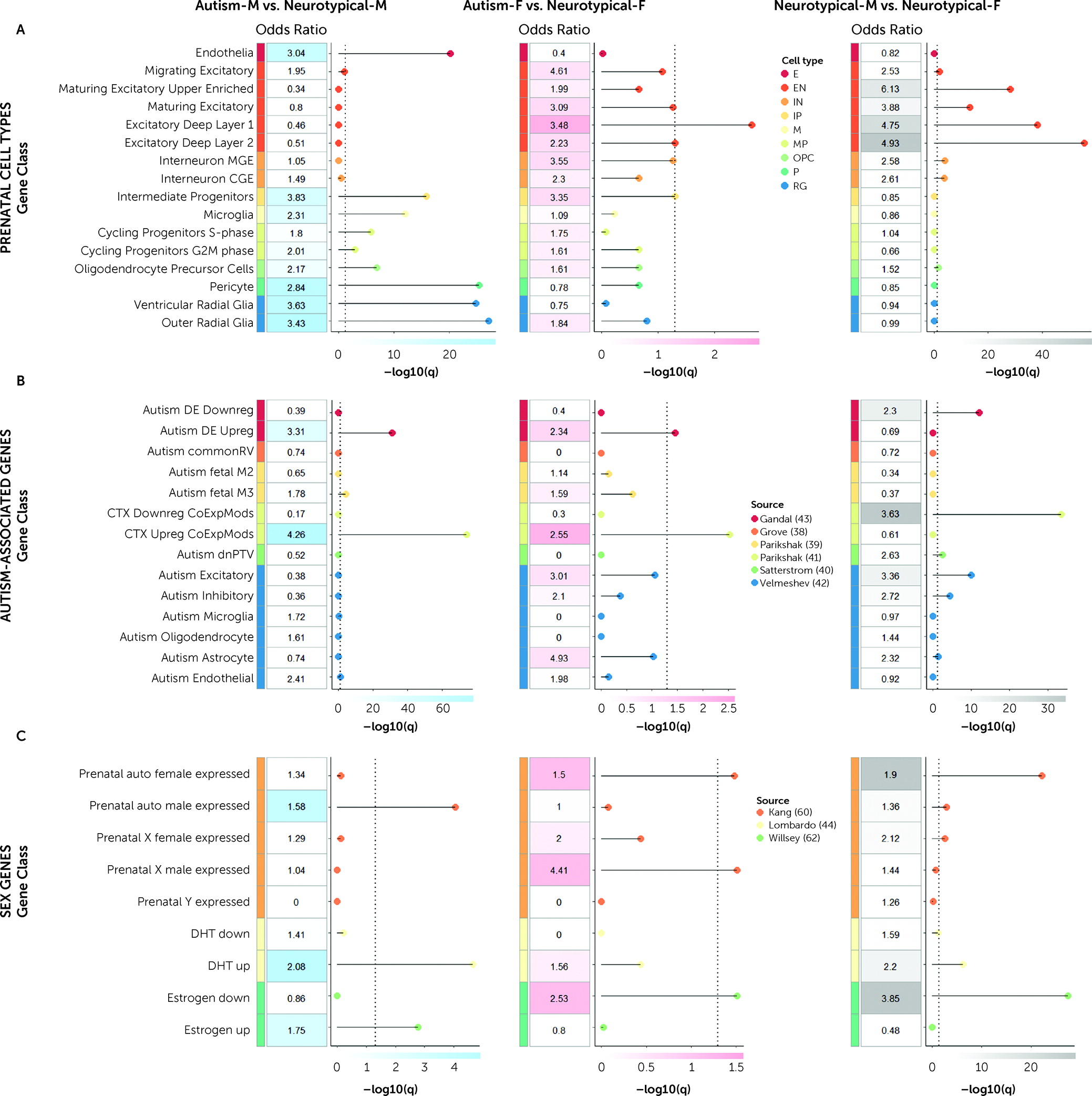
We further observe that genes relevant to male-shifted regions in autistic males are enriched both for genetic markers of intermediate progenitor (IP) and radial glial (RG) cell types, as well as genes upregulated by DHT and estrogen. In line with this, it has been shown that DHT increases proliferation of IP and RG cell types (
92). Also, differences in proliferative processes through symmetric cell division in IP and RG cell types lead to atypical cortical expansion of surface area in autism (
56,
93,
94). These downstream consequences of DHT may impact excitatory neuronal signaling, due to expansion of surface area via increased numbers of cortical columns and neurons within each column. Furthermore, genes involved in excitatory postsynaptic potentials and autism-associated genes affecting excitatory neuronal lineages are dysregulated by DHT (
44,
54), and consequently, excitation-inhibition imbalance is asymmetrically more affected in autistic males (
54).
On the other hand, in autistic females, male-shifted regions are particularly enriched for later differentiated excitatory neurons and genes downregulated by estrogen, but not for genes upregulated by DHT (as in autistic males). This is in line with clinical findings indicating that autistic females may be characterized by a relative imbalance between androgens and estrogens, rather than high steroid levels across all pathways (
95,
96). Conditions related to androgen/estrogen imbalances in females (e.g., polycystic ovary syndrome) have previously been linked to autism likelihood in both mothers and their children (
97–
100). Further, estrogen-regulated signaling can influence region-specific neurodevelopmental male shifts and affect neuronal excitation (
101–
103), but more studies are needed to demonstrate this in human-derived cell models. Thus, the enrichment effects in IP, RG, and DHT in autistic males and in excitatory neuronal cell types and estrogen in autistic females may be relevant to the overall idea that one key emergent phenomenon of brain masculinization is the effect it has on excitation-inhibition imbalance (
104), with potentially different underlying sex-steroid-related mechanisms in autistic males and females (
90).
Furthermore, microglial neuroimmunological processes may be major contributors to brain masculinization (
14). Werling et al. (
59) reported enrichment of male-biased microglial expression with autism-upregulated coexpression modules along with enrichment of female-biased synaptic expression with autism-downregulated coexpression modules, corroborating that molecular downstream pathways regulating neurotypical male development interact with those of autism-associated genes. These observations are consistent with our discoveries that 1) genes expressed in regions overlapping with male-shifted regions in both autistic males and autistic females are enriched for upregulated autism-associated genes mapping onto inflammatory pathways (
41) (likely underlying vulnerability mechanisms associated with males [
105]), 2) regions differentiating neurotypical females from neurotypical males are enriched for autism-downregulated expression modules (likely underlying protective mechanisms associated with females [
105]), 3) the male-shifted regions in autistic males are enriched for prenatal microglial cell types, and 4) downstream transcriptionally dysregulated pathways are more involved in sex-differential processes than they are in upstream genetic susceptibility mechanisms.
Our findings of reduced and superior sex prediction performance in autistic females and males, respectively, are most pronounced in childhood and decrease throughout development to young adulthood. In the age bin of 20–30 years, we do not observe classification differences across groups. Similarly, two other studies in adult samples with mean ages of 22 years (
7) and 26 years (
5) also showed no sex prediction accuracy differences between autistic and neurotypical adults. It is likely that these age-related patterns are influenced by dynamically unfolding interactions between sex-differential and neurodevelopmental process across the lifespan. Sex differentiation emerges in utero, and many on-average sex differences persist into adulthood, while others are transient and context dependent, meaning they become apparent at specific developmental stages depending on factors such as hormonal milieu and experiences (
71). For example, sex steroid hormones are thought to initially act organizationally during perinatal development, and then activationally during puberty to influence the formation and expression of sex differences at different developmental windows (
106). Clinically, the rate of pubertal differentiation was, for example, found to interact with the association of autistic traits with fetal testosterone levels in a longitudinal cohort (
107). Also, different molecular mechanisms are highly region specific, and they are again expressed at different developmental periods (
71). One example of an age-dependent neurophenotype in autism is early brain overgrowth, which is more likely observed in autistic children but not later in life (
108,
109). Atypical age-related cortical development has also been demonstrated by deviations from the typical developmental trajectories both in cortical thinning in autistic children and adolescents in longitudinal studies (
110–
112) and in neuronal differentiation in in vitro models (
113). Here, we identified associations with prenatal cell types that have been linked to atypical cortical expansion in autism (
56) and likely link back to sex-specific excitation-inhibition imbalance across neurodevelopment. On top of these sex-related mechanisms, gendered experiences, gender role expectations, and gender socialization based on one’s sex assigned at birth in a society may also influence neurobiology and postnatal brain development (
2,
114). Considering this mechanistic complexity, future studies (especially longitudinal ones) need to unravel the observed spatiotemporal sex-differential profiles in autism and establish how experiential, environmental, genetic, and hormonal effects interact to differentially contribute to the observed patterns across different developmental windows (e.g., fetal/neonatal stage, childhood, puberty/adolescence, adulthood/reproductive age, and old-age/postmenopause). Finally, we cannot exclude nosological issues and ascertainment bias contributing to the observed age-related patterns. Autism diagnostic practices have been described as male biased (
3). It is thus possible that females presenting with “typical/classical” (i.e., male-like) autistic features (which might be related to the observed neuroanatomical patterns) may be diagnosed earlier in life than females presenting with more nuanced phenotypes that are less well captured by current diagnostic practices (
115), hence contributing to the age differences in classification performance. We have no available data on age at diagnosis in the present study; however, future studies should disentangle the effects of chronological age and timing of autism diagnosis.
Finally, it has been suggested that the male preponderance in neurodevelopmental conditions may be driven by similar genetic and neuroendocrinological mechanisms. Here, we show that autism is associated with a different sex-differential neuroanatomical profile in males and females than is ADHD. A proposed pathway through which sex-differential prevalence and presentation in ADHD is mediated may be atypical dopaminergic system function modulated by gonadal hormones (
116,
117), such as an increase in the striatal dopamine receptor density in prepubertal development in males but not females (
118). Future research needs to pinpoint which exact sex-related mechanisms shape differential neurophenotypes in different male-biased conditions.
Strengths and Limitations
Our analytical approach has multiple strengths. We employ four of the largest cohorts available for our populations of interest (neurotypical, autism, ADHD), thus ensuring both replicability and specificity of findings. We carefully address potential confounders, confirming that the results are not driven by differences in brain volume, age, and model choice. Our analyses do not rely on artificial features, but by employing a novel deep convolutional neural network model with superior performance (
46), we take whole-brain anatomical data as predictive features. Nevertheless, there are several limitations. Even though our samples span wide age ranges and the results imply the likely involvement of neurodevelopmental mechanisms, only when investigating a longitudinal cohort will we be able to make causal inferences. Further, we did not address social, cultural, or experiential factors (
114) that can influence sex-differential brain development (
71), or gender identity, which might differ from sex assigned at birth, especially in autistic individuals (
119). The data sources employed in the current study did not gather information specifically distinguishing sex at birth and gender identity. As such, the potential for misspecification of participants’ sex at birth exists, but the rate is anticipated to be low. Within this limitation, given that our models were trained to predict biological sex, we used the term sex-specific prediction accuracy. In addition, while utilizing a highly accurate approach for sex classification, the high level of nonlinearity intrinsic to the convolutional neural network model makes straightforward interpretations more difficult compared to simpler linear models. We address this issue by a novel approach for model introspection by means of region-aligned prediction. This approach has the advantage of being able to generate spatially resolved estimates of sex-specific accuracy prediction, but due to the convolutional structure of our model, it achieves this with lower spatial fidelity than the original input data. Yet, being able to spatially resolve the sex prediction accuracy enables the further association analyses with cognition and gene expression. We further note that our enrichment analyses are a correlational approach that cannot reveal the causal genetic mechanisms of our brain structural findings. However, this approach can highlight genes for further molecular analyses to establish mechanisms and directionality of findings (
23). Also, we only tested a select number of genes for which there is a prior literature implicating them as robust markers of autism likelihood, prenatal development, and sex-differential biology. While we also acknowledge the methodological limitation of using adult brain expression data in association with prenatal cell types, our results provide important signals that neuroanatomical male shifts in autism are associated with genes that are predominantly expressed in prenatal development and may therefore affect neurodevelopmental processes. Also, the Allen Human Brain gene expression atlas is the most comprehensive and highly sampled gene expression atlas currently available—albeit one based on adult donors only. Once high-resolution, whole-brain, and age-specific gene expression maps become available, future studies should investigate gene expression profiles in the developing human brain. This will further elucidate the genes associated with autism-related sexual differentiation acting at different developmental windows.
Acknowledgments
The authors thank all participants and their families for participating in the studies that contribute to the data sets used in this research. The authors also gratefully acknowledge the contributions of all members of the EU-AIMS/AIMS-2-TRIALS LEAP group: Jumana Ahmad, Sara Ambrosino, Bonnie Auyeung, Tobias Banaschewski, Simon Baron-Cohen, Sarah Baumeister, Christian F. Beckmann, Sven Bölte, Thomas Bourgeron, Carsten Bours, Michael Brammer, Daniel Brandeis, Claudia Brogna, Yvette de Bruijn, Jan K. Buitelaar, Bhismadev Chakrabarti, Tony Charman, Ineke Cornelissen, Daisy Crawley, Flavio Dell’Acqua, Guillaume Dumas, Sarah Durston, Christine Ecker, Jessica Faulkner, Vincent Frouin, Pilar Garcés, David Goyard, Lindsay Ham, Hannah Hayward, Joerg Hipp, Rosemary Holt, Mark H. Johnson, Emily J.H. Jones, Prantik Kundu, Meng-Chuan Lai, Xavier Liogier D’ardhuy, Michael V. Lombardo, Eva Loth, David J. Lythgoe, René Mandl, Andre Marquand, Luke Mason, Maarten Mennes, Andreas Meyer-Lindenberg, Carolin Moessnang, Nico Mueller, Declan G.M. Murphy, Bethany Oakley, Laurence O’Dwyer, Marianne Oldehinkel, Bob Oranje, Gahan Pandina, Antonio M. Persico, Barbara Ruggeri, Amber Ruigrok, Jessica Sabet, Roberto Sacco, Antonia San José Cáceres, Emily Simonoff, Will Spooren, Julian Tillmann, Roberto Toro, Heike Tost, Jack Waldman, Steve C.R. Williams, Caroline Wooldridge, and Marcel P. Zwiers. The authors also gratefully acknowledge the contributions of all members of the APEX (Autism and Prenatal Sex Differences) group: Dwaipayan Adhya, Carrie Allison, Simon Baron-Cohen, Rosemary Holt, Tracey Parsons, Paula Smith, Alex Tsompanidis, Varun Warrier, Graham J Burton, Kathy K. Niaken, David H. Rowitch, Alexander E.P. Heazell, Lidia V. Gabis, Tal Biron-Shental, Madeline A. Lancaster, Deepak P. Srivastava, Jonathan Mill, Matthew E. Hurles, Hilary C. Martin, and Daniel H. Geschwind. The authors thank Dr. Steve Smith, Dr. Nicolas Langer, and Dr. Amber Ruigrok for helpful comments and feedback on the manuscript.