The National Institute on Drug Abuse (NIDA)—first established in 1974 as part of the Alcohol, Drug Abuse, and Mental Health Administration and in 1992 becoming part of the National Institutes of Health (NIH)—is the largest funder of research on drug use and addiction in the world. Over its 50-year history, NIDA’s work has led to tremendous progress in advancing science and improving individual and public health. But the overdose epidemic at hand is a powerful reminder that there is much more to be done. Commemorating NIDA’s 50th anniversary provides an opportunity to acknowledge the significant advances that have been made in addiction science, as well as take stock of the remaining gaps and challenges and affirm our commitment to championing research that can improve the lives of those who are at risk or suffer from a substance use disorder (SUD).
When I look back at 50 years of NIDA research, I am stunned at how far the science of addiction has advanced and at the contributions NIDA-supported researchers have made. When the Institute was founded, our scientific field was in its infancy. The mu-opioid receptor had just been discovered, but the endogenous opioid signalers were not yet identified and the central role of dopamine in reinforcement and motivation and its role in addiction had yet to be elucidated. While disulfiram and methadone had been approved for treating alcohol and opioid addiction respectively, there were no other pharmacotherapies for drug use disorders, and there was little in the way of effective behavioral treatments or effective prevention programs.
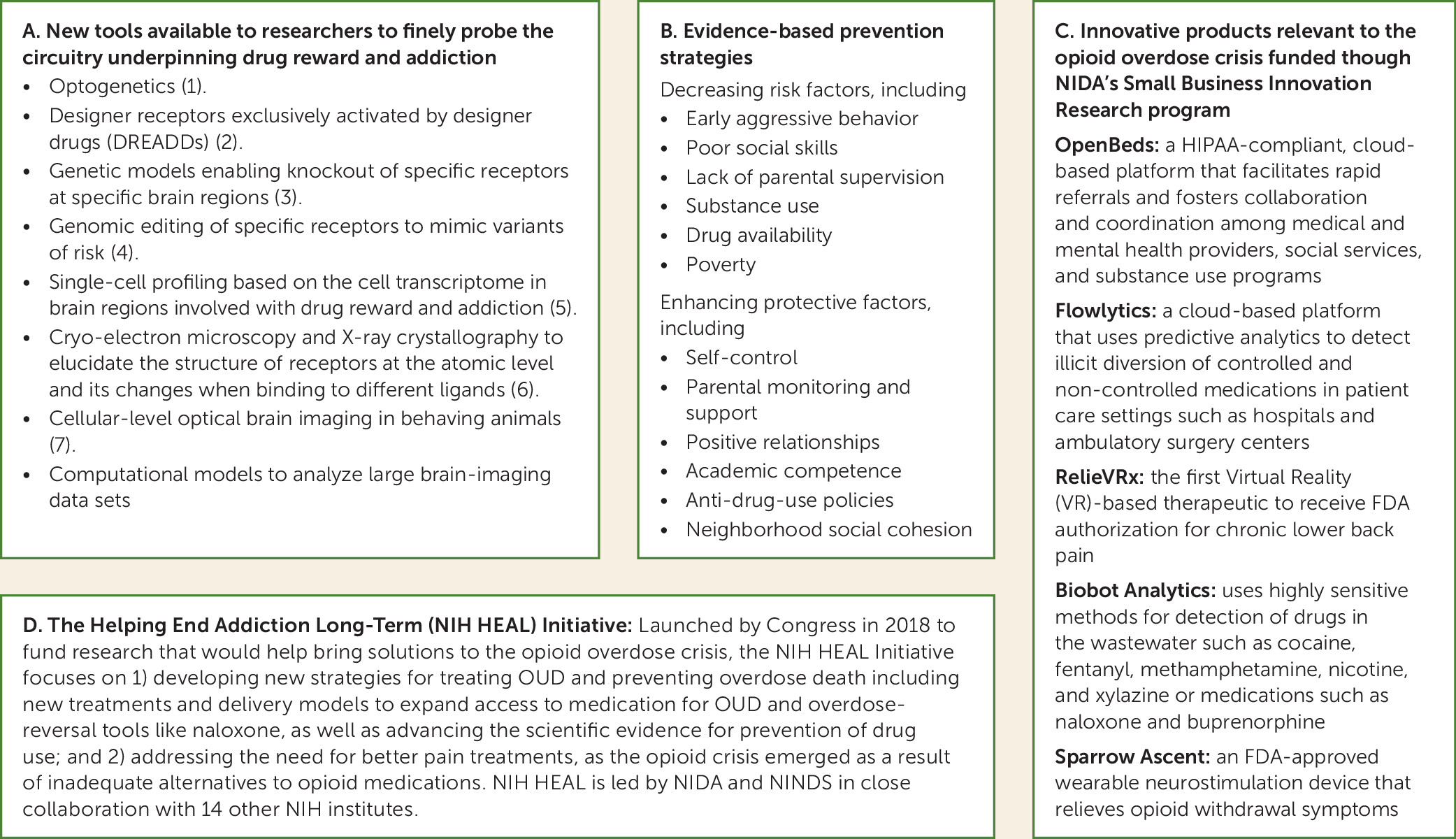
Over the intervening decades, our understanding of the pharmacology of drugs and the neurotransmitter systems mediating their effects has vastly increased. NIDA-funded researchers including those at NIDA’s Intramural Program have leveraged increasing advances in molecular, neuroimaging, and computational tools (
Figure 1, panel A) (
1–
7) to develop a much more nuanced understanding of the complex signaling and neurocircuitry underlying drug reward and addiction. Thanks to this research, addiction is now recognized as a disorder that results from drug-induced neuroplastic changes in multiple brain networks including those involved in reward, executive function, emotion processing, and interoceptive awareness.
Epidemiological studies had shown decades ago that social determinants of health, most notably adverse childhood experiences, significantly increased the risk for drug use and addiction. But researchers have now started to characterize how these adverse exposures influence the development and function of the human brain. Similarly, while it was recognized that addiction ran in families, presumably reflecting genetic influences, researchers have now identified gene variants that influence substance use, addiction, and drug treatment outcomes and that might mediate environmental effects (
8).
Meanwhile, prevention research conducted over the past five decades has led to evidence-based interventions that significantly reduce drug use and the progression to SUD (
9). Evidence-based prevention interventions for SUD are based on the premise of decreasing risk factors while enhancing protective factors (
Figure 1, panel B) through individual, family, school, or community interventions. In addition to reducing drug use, some of these interventions have buffered detrimental effects of poverty on brain development (
10), whereas others have shown benefits in reducing drug use even in the children of parents who had received the intervention during childhood (
11).
Yet, despite the existence of effective prevention interventions, and despite their cost-effectiveness, they are not widely utilized. NIDA is supporting research to test prevention interventions in service delivery settings including schools, human services, and healthcare, where there is greater potential for uptake, scalability, and sustainability (
12). We are also collaborating with our federal agency partners to ensure that prevention becomes a greater priority for policymakers and communities and that we have the critical infrastructure needed to provide sustainable access to prevention interventions (
13).
In the field of therapeutics, NIDA research has advanced the development of behavioral interventions, medications, immunotherapies, and neuromodulation. Behavioral research has expanded our understanding of cognitive processes underlying addictive behaviors, including impulsivity, risk perception, and reward processing, which has led to advances in behavioral treatments for addiction. For example, research on behavioral economics propelled the development of contingency management, which reinforces positive behavior change such as reduced drug use or abstinence and is currently the most effective behavioral treatment for psychostimulant use disorders. Similarly, motivational interviewing benefited from research on the influence of interpersonal processes on behavioral change to strengthen the motivation and commitment to reduce or avoid drug taking (
14).
Medication development has also benefited from NIDA funded research. Nicotine replacement therapy for smoking cessation was developed with NIDA support, for example; and particularly impactful for the current overdose crisis was the research that led to the development and FDA approval of buprenorphine and naltrexone for opioid use disorder (OUD). These are highly effective medications for reducing opioid use and preventing overdoses (
15), and they greatly expanded the settings where patients can be treated beyond specialty methadone programs, such as primary care and psychiatric clinics. NIDA research has also substantiated the effectiveness of methadone in OUD treatment, and it was instrumental in the development and FDA approval of nasal naloxone, a medication for overdose reversal that is easily delivered by laypersons and is now saving thousands of lives every year.
Medications still do not exist for addiction to substances other than opioids, nicotine, and alcohol. NIDA is actively pursuing scientific opportunities that promise to fill these gaps for the treatment of stimulant, cannabis, and polysubstance use disorders. Using methods as described in
Figure 1, panel A alongside high-resolution dynamic models, drug targets are now computationally screened against virtual libraries of millions of compounds, greatly accelerating the process of drug discovery. NIDA’s medication-development portfolio now includes dozens of novel compounds and potentially revolutionary non-medication approaches (
16).
Immunotherapies, including vaccines and monoclonal antibodies, are among the innovative approaches being pursued. The strategy is based on the generation of antibodies that bind to the drug in the blood to interfere with its entry into the brain or other tissues. Vaccines offer a longer duration of effects than monoclonal antibodies, but the titers needed for efficacy, for the clinically tested vaccines, have thus far been insufficient. Direct delivery of antibodies leads to higher titers, and new advances in their design confers longer half-lives on them.
Also of great interest are neuromodulation approaches that target the brain circuits implicated in addictive behaviors. These could offer the possibility of personalizing the intervention to an individual brain functional deficit to be used by themselves or in combination with medications or behavioral interventions (
17). Active efforts to accelerate the translation of scientific findings into tools or products have led to a range of devices and apps for preventing, diagnosing, and treating addiction and its health effects (
Figure 1, panel C shows examples of products currently on the market).
Maximizing the benefits from evidence-based therapeutic interventions for SUD has required research on new models for deploying these treatments in health care and justice settings, and for their implementation in diverse communities. Countering the stigma and misinformation that have impeded treatment efforts is part of this research effort.
NIDA’s Clinical Trials Network, established in 1999, has led to expanded engagement of various healthcare providers in the screening and initiation of medications for OUD, in the deployment of behavioral treatments like motivational interviewing, and in integrated treatment approaches for people with addiction and other co-occurring mental illnesses. NIDA-funded research in justice settings has shown that initiation of methadone or buprenorphine prior to release improves outcomes including post-release treatment engagement (
18,
19). Providing medication to this population is critical, as many people in jails and prisons have OUD and, without treatment, are at very high risk of overdose upon release. The Justice Community Opioid Innovation Network (JCOIN), funded though the NIH HEAL initiative (
Figure 1, panel D), is developing models for OUD treatment in these settings; JCOIN studies have shown that expanded access to medications for OUD in prisons and jails could reduce overdose deaths by 30% (
20) and decrease recidivism (
21).
Also in line with the urgency to address the current overdose crisis, implementation science is prioritizing other strategies for expanding access to methadone, buprenorphine, and naltrexone treatment for OUD, since fewer than 20% of people who could benefit receive them (
22). Examples include initiating buprenorphine through mobile clinics, via telehealth, and in emergency departments following nonfatal overdoses. During the COVID pandemic, the expanded prescription of buprenorphine through telemedicine ensured the continued treatment of patients, and telemedicine has continued to facilitate OUD treatment initiation and retention.
NIDA research on the intersection of substance use and HIV has been transformative for HIV care. For example, NIDA‐funded research supported the approach of HIV treatment as prevention and demonstrated the benefits of SUD treatment for improvement of HIV outcomes (
23). And through its International Program, NIDA-funded researchers have contributed greatly to the expanded acceptance of methadone treatment as HIV prevention and as a strategy to improve clinical outcomes in HIV+ individuals who have OUD.
As we look into the future, we can predict that the landscape of substance use will continue to change, including the emergence of new and increasingly more powerful synthetic drugs, as well as state-level legislative changes in medicinal and recreational use of drugs like cannabis. Consequently, research should be able to inform public health and policymakers on these developments. This will benefit from more sensitive and accessible tools for detection of new compounds in the drug supply, as well as continued research on how cannabis, psychedelics, or other drugs marketed to consumers affect health, including their potential value as therapeutics.
The data revolution has already started to make it possible to investigate very complex systems, which in the case of drug use and addiction requires studying the intersection between genetics and neurocircuitry and how our brains and physiology dynamically interact with the environments in which we live. The ongoing NIDA-led Adolescent Brain Cognitive Development longitudinal study of nearly 12,000 teens has already yielded tremendous insights into the neurodevelopmental impacts not only of drugs but also of poverty and racial discrimination, social media and screen time, and a wide range of other factors affecting youth during a crucial period of maturation (
24). Newly underway is the NIDA-led HEALthy Brain and Child Development study, which will measure neurodevelopment longitudinally starting in infancy and throughout the first decade of life (
25).
Increasingly large data sets and powerful computational tools and algorithms are enabling us to move from very focused, simplified scientific questions toward ever more comprehensive and dynamic ones. However, realizing science’s potential to improve people’s well-being and health will require that we ensure that it does not contribute to the deep racial/ethnic and sex/gender health inequities that exist in the field of addiction. Thus, we have to continue to expand the involvement of researchers from underrepresented groups, actively engage people with lived experiences, and promote research to inform policies that ensure wide access to evidence-based prevention and treatment interventions and harm-reduction services.
A half century of NIDA research has shown clearly that SUDs are chronic but treatable brain disorders that emerge from the complex interplay of biological, social, and developmental factors. No matter their background or circumstances, people with these conditions deserve compassionate, high-quality, evidence-based care, offered in an accessible and practical way. The continued losses from overdoses are forefront in our minds as we vigilantly seek novel scientific and policy solutions to end the current crisis and support people’s recovery from addictive disorders that we know—because the science shows it—is achievable.
Acknowledgments
The author acknowledges the editorial assistance of Jennifer Hobin, Emily B. Einstein, and Eric M. Wargo in preparing this article.