Functional neurological disorders (FNDs; or psychogenic neurological symptoms), also known as conversion disorder, are a classic neuropsychiatric disorder, existing in the border between neurology and psychiatry. Unexplained neurological symptoms are common, occurring in up to one-third of patients in neurological outpatient clinics.
1 Relative to disorders such as Parkinson’s disease, FND is associated with similar levels of disability and physical quality of life with poorer mental health quality of life.
2 The prognosis is poor, with a recent review showing that up to 40% of patients with FND report similar or worse outcomes at 7-year follow-up.
3 Given the frequency, consequences, prognosis, and burden, FND has been called a “crisis for neurology.”
4 Despite this, FND is poorly understood. In the last decade, an increasing number of studies have focused on underlying neurobiological mechanisms.
FND is defined in
DSM-5 as the presence of one or more symptoms of altered voluntary or sensory function, with clinical findings providing evidence of incompatibility between the symptom and recognized neurological or medical conditions.
5 The
DSM-5 diagnosis differs from that of the
DSM-IV diagnosis of conversion disorder by adding the criterion of incorporating physical diagnostic features and by relegating to the accompanying text the criteria requiring (a) an association with psychological stressors and (b) the exclusion of malingering or factitious disorder to make the diagnosis.
DSM-5 FND diagnosis criteria now allow for potential greater interrater reliability, making them appropriate for research studies and greater compatibility with specialty-specific diagnoses including psychogenic movement disorder (PMD) and psychogenic nonepileptic seizures (PNES).
6The putative biopsychosocial mechanisms underlying FND are complex and have been extensively reviewed.
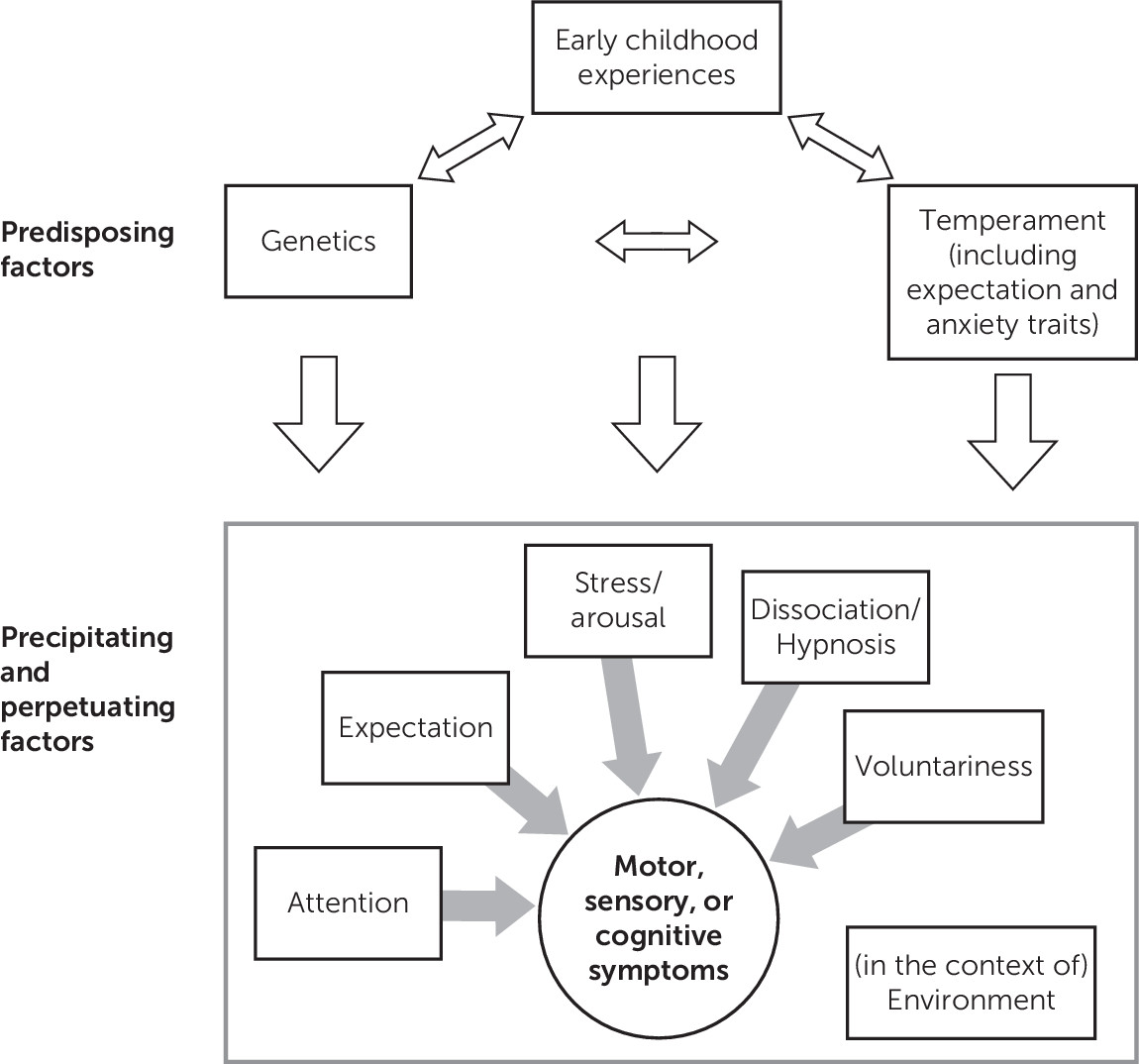
7 Our review focuses on the physiological mechanisms that may underlie FND and does not specifically focus on the extensive literature on the antecedents of early childhood experiences and temperament (
Figure 1). At the outset, regarding the proposed model, we acknowledge that predisposing factors for functional neurological symptoms are not limited to genetics, temperament, and early childhood experiences. A number of other relevant issues are contributors to the presence of conversion symptoms. Along with the neurophysiologic markers (e.g., cortical network, autonomic, and psychophysiologic) reviewed in this article, psychodynamic theory (unconscious conflict with somatic symptom presentation) and learning theory (maladaptive coping associated with illness behaviors) are part of the formation of FNDs; however, there are limited neurophysiologic studies quantifying these etiologic hypotheses.
7 The locations of the factors listed in
Figure 1 are not limited to predisposing, precipitating, and perpetuating factors. We have merely identified component contributors (not in a structural, mechanistic manner). For example, along with being a perpetuating factor (as seen in the fear-avoidance model), we have included expectation as a predisposing factor. Where possible, this review indicates whether the findings focus on all forms of FND or more specific subtypes.
This review is divided into the following sections (depicted in the second part of
Figure 1). The first section reviews physiological, neural function, and anatomical correlates subdivided into motor, sensory, and visual function and PNES. The second section reviews the role of attention, cognitive function, expectation, voluntariness, dissociation, hypnosis, arousal, and trauma. The final summary provides recommendations for subsequent studies.
There are several caveats in the interpretation of this review. Most of the studies have small sample sizes as a function of the difficulties in recruitment of participants. Given the length of the review, key points are summarized and each study paradigm cannot be explained in depth; hence, readers are referred to the source document for design details. Although the majority of FNDs originate from psychosocial developmental or traumatic precursors, there is possible heterogeneity in the mechanisms leading to various symptom expressions. Furthermore, different presentations or semiologies may have differing neural correlates.
In this review, several terms will be used that describe variations of somatoform disorders, including functional disorder, FND, PMD, and nonepileptic seizures. We realize that use of the term “functional” has an evolving meaning in neuropsychiatric history. “Functional” was originally used in contrast with gross neuroanatomic structural pathology. In this article, we propose that the term “functional” may account for symptoms involving neural networks mediating both neurophysiologic and intrapsychic processes.
Search Terms and Strategy
The following search terms were used in MEDLINE and non-MEDLINE searches, yielding 2,556 and 1,596 citations, respectively: (functional disorder OR psychogenic OR somatoform disorder OR somatization) AND (physiology OR pathophysiology OR cortisol OR cognition OR imaging OR neuroimaging OR MRI OR fMRI) (English only). The following search terms were used in MEDLINE and non-MEDLINE searches, yielding 110 citations: (nonepileptic seizure OR psychogenic seizure OR nonepileptic attack disorder OR pseudoseizure) AND (neuroimaging OR physiology OR pathophysiology OR cortisol OR cognition OR MRI or fMRI) (English only). The search term “psychogenic movement disorder” alone was used to ensure there were no missed articles, yielding 392 citations. Abstracts from all citations identified in the search were reviewed, and those relevant to the neurophysiology of FND were reviewed. References from articles reviewed that were not identified in the original online search but were found in reviews of chapter references were also included.
Physiological and Neural Function and Anatomical Correlates
The differing presentations of FND have been studied, including motor, sensory, visual, and PNES, using modalities such as neurophysiology, serum biomarkers, cognitive tasks, and functional and anatomical imaging. Motor presentations can be subdivided into negative symptoms (i.e., weakness or paralysis) or positive symptoms (i.e., psychogenic movement symptoms such as tremor or dystonia, or motor seizures). Whether these symptom presentations have similar underlying commonalities on a neurophysiological level (i.e., How are these symptoms related?) and how they might differ (i.e., Why do individuals present with differing symptoms?) remain important unanswered questions. Here we subdivide this review by symptom presentations.
Motor
The question of an impairment in motor function in functional paralysis was first raised with early imaging studies asking whether (a) the upstream process of motor conceptualization, action selection, or initiation was impaired or more downstream effects at the level of execution was impaired,
8 or (b) if a generalized inhibitory process may play a role in inhibiting execution.
9 These questions parallel the literature in hypnotic paralysis, as discussed in the section on dissociation.
10–12Conceptualization and initiation.
In an early small positron emission tomography (PET) study, Spence et al.
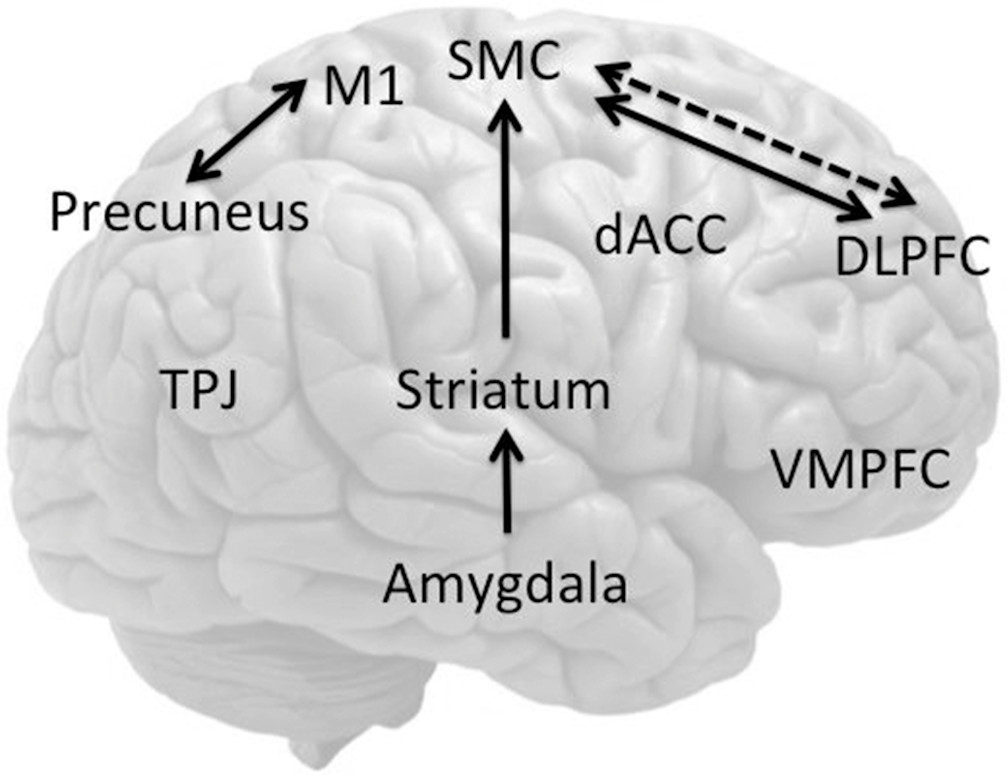
8 showed that joystick movement in unilateral functional paralysis (N=3) was associated with decreased left dorsolateral prefrontal cortex (DLPFC) activity, regardless of the side of the deficit. By contrast, feigned paralysis was associated with decreased right anterior prefrontal cortex activity (
Figure 2). The authors suggest that the left DLPFC hypoactivity represented a possible impairment in higher-order internal generation or conceptualization of action. Spence et al.
8 postulated a disturbance of volition and disorder of will, as follows: “the patient [says] ‘I cannot’; it looks like ‘I will not’; but it is ‘I cannot will’ (Paget, 1873).” These findings implicating the DLPFC have not been replicated in another small study of a similar design assessing attempted movement in functional paralysis,
13 although other regions (particularly the striatum
13 and parietal cortex
14) have been implicated, as discussed below (
Table 1).
Several lines of study have since attempted to isolate motor conceptualization from execution, implicating impairments in the process of conceptualization. Roelofs et al.
15 showed longer reaction times in both “explicit” and “implicit” motor rotation tasks among patients with functional paralysis (N=6) compared with healthy volunteers. In the implicit rotation task, participants made judgments of whether images of rotated hands and feet were left or right. In the explicit rotation task, participants were explicitly asked to mentally rotate their own hands or feet to match the target position of the rotated image before making the same judgment. In the explicit rotation task, individuals with functional paralysis were slower overall in mental rotation, with greater impairments in the affected compared with the unaffected side of the body, whereas reaction time measures of implicit rotation were intact. Patients with functional paralysis subjectively reported that they could not explicitly mentally rotate their hands or feet to match the image in 51% of foot trials compared with 0% in controls. The authors suggest that the general slowing of motor initiation is attributable to impaired explicit intentional processes, whereas implicit conceptual processes were intact.
15 In a separate analysis of the same study, functional paralysis (N=4) was associated with greater impairment in indices of motor initiation or reaction time but not in indices of execution times or response duration, again suggesting impairment of explicit initiation.
16Using motor imagery or movement observation tasks in imaging studies of functional paralysis allows indirect assessment of neural functioning without the confounder of neuroanatomically mediated motor impairment. Burgmer et al.
17 showed (N=4) decreased activity in motor regions of patients with functional paralysis during movement observation contralateral to the side of the paralysis but no differences in activity in motor inhibitory areas; the authors suggested that these findings support an abnormality of movement conceptualization. de Lange et al.
18 showed that implicitly induced motor imagery of the affected hand recruited ventromedial prefrontal cortices (VMPFCs) and superior temporal cortices of patients with unilateral functional paralysis (N=8) compared with the unaffected hand, suggesting heightened self-monitoring (
Figure 2). The VMPFC, superior temporal cortex, and precuneus are implicated in a default mode network, which has been shown to be more active at rest with online decreases during cognitive processes or action.
19 In a follow-up study, de Lange et al. showed that, compared with the unaffected hand, implicit (but not explicit) motor imagery of the affected hand was associated with increased activity in the VMPFC.
20 The authors suggested that this was related to the greater cognitive load in explicit imagery, which may play more of a role in suppressing default mode network activity. Similarly, Czarnecki et al.
21 compared functional tremor (N=5) with essential tremor (N=5) and healthy controls using single photon emission computed tomography (SPECT) at rest and during a tremor-inducing motor task (to bring a cup from a table to the face). During the motor task, patients with functional tremor had decreased regional cerebral blood flow (rCBF) in the VMPFC consistent with abnormalities of the default mode network, which were not observed in essential tremor (
Figure 2). Both patients with essential tremor and healthy controls activated the supplementary motor area (SMA) and deactivated the visual cortex, whereas individuals with functional tremor activated the cerebellum. In the same study by de Lange et al.,
22 the authors showed greater functional connectivity (FC) of the affected hand, compared with the unaffected hand, between the left DLPFC and sensorimotor regions, including the SMA.
Focusing on the motor intention phase of movement, Voon et al.
23 showed decreased activity in the SMA and increased activity in limbic regions (amygdala, anterior insula) among patients with functional motor symptoms (N=11) compared with healthy volunteers (
Figure 2). During the cue for freely chosen actions (to press either one of two buttons) compared with cued actions (cue for specific button press), individuals with FND had decreased FC between the DLPFC and SMA, suggesting a possible impairment in higher-order action selection, particularly during freely chosen actions. The FC findings are consistent with the findings by de Lange et al., in that freely chosen voluntary actions are associated with a decrease in DLPFC-SMA FC compared with cued voluntary actions. By contrast, cued implicit motor imagery of the affected arm is associated with the opposite increase in DLPFC-SMA FC.
Edwards et al.
24 further investigated this subjective urge to move using the Libet’s clock paradigm, in which patients with psychogenic tremor (N=9) made voluntary movements while watching a rotating clock. Participants reported the time on the clock in which they felt the urge or intention to move (W) and the time in which the actual voluntary movement occurred (M). The subjective conscious awareness of the intention (W) was judged to occur much later in patients with psychogenic tremor than in controls, suggesting that the sense of volition during voluntary movements might be impaired and experienced as involuntary.
24The SMA is a crucial region in intentional processes. In healthy volunteers, the SMA (SMA proper and pre-SMA) is implicated in the subjective urge to move and the intention to move. For instance, in healthy volunteers (tested using the Libet’s clock in which individuals were asked to either pay attention to the “urge” or intention to move or the movement itself), paying attention to the intention to move is associated with greater blood-oxygen-level–dependent (BOLD) activity in the pre-SMA, DLPFC, and intraparietal sulcus.
25 Attention to intention, compared with movement, is also associated with greater FC between the DLPFC and pre-SMA. Likewise, among patients with epilepsy, stimulation of the SMA was associated with the urge to move.
26 A gradual increase in SMA single-unit activity precedes the onset of the conscious intention to move (W) by up to 1,500 ms. Furthermore, using machine learning classification techniques, activity in SMA neurons can predict the decision to move at 700 ms before the awareness of movement with 80% accuracy.
27 These studies highlight that among patients with FND, the DLPFC-SMA may be engaged in aberrant intentional processes during implicit motor imagery of the affected arm but fails to be engaged during voluntary, freely chosen movements.
Taken together, these findings suggest a possible impairment in motor conceptualization or intention during both voluntary movements and motor imagery of the affected limb, possibly implicating a DLPFC-SMA network in FND.
Inhibition.
In contrast with conceptualization of motor execution theory, a series of studies have focused on the question of an “inhibition” of motor execution (
Table 2). This approach was first proposed by Marshall et al.
9 in a classic case study of functional paralysis. The patient showed intact lateral premotor and cerebellar regions with the preparation to move and activated left DLFPC and cerebellar regions but not other motor regions with attempted movement. The contrast of attempted movement versus preparation was associated with increased right anterior cingulate and orbitofrontal cortex (OFC) activity. On the basis of these findings, the authors suggested that preparation to move is intact but that execution is inhibited by prefrontal regions (
Figure 2).
9Inhibitory processes can be measured using neurophysiology, motor inhibition tasks, or neuroimaging, as described in the following studies.
The motor evoked potential (MEP) has been used to show intact corticospinal excitability in functional paralysis both during acute functional paralysis
28,29 and after resolution of symptoms.
30 Morita et al.
31 showed that in response to the signal cue to move, the MEP had greater variance in patients with functional paralysis (N=10) compared with patients with amyotrophic lateral sclerosis and healthy controls. Liepert et al.
32 showed that in response to motor imagery, patients with unilateral functional paralysis (N=5) or fixed dystonia (N=3) had a decrease in MEP of the affected hand by 37%, compared with rest with an increase in MEP by 63% of the unaffected side These findings were specific to MEPs because motor threshold, short intracortical inhibition, and intracortical facilitation were shown to be unremarkable among individuals with functional paralysis.
33,34In healthy individuals, motor imagery commonly increases MEPs to a level comparable to that observed during executed movements. In a study by Liepert et al.,
34 subjects were asked to imagine a tonic adduction of the affected index finger, the unaffected index finger, and both fingers simultaneously with the transcranial magnetic stimulation (TMS) pulse applied 2 seconds after the imagery signal. Although movement imagination was not objectively assessed, the authors note a decrease relative to rest in individuals with functional paralysis, suggesting an active process of inhibition during the task. The authors suggest that these changes resemble MEP changes in healthy volunteers during negative motor imagery. For instance, in healthy volunteers, imagination of suppression of the TMS-induced twitching to the no-go signal, compared with the “no imagination” state, was associated with a decrease in MEP amplitude, as opposed to imagination of squeezing hands to the go signal.
35 Thus, Liepert et al. suggest that their findings might imply a similar inhibitory process during motor imagery in functional paralysis resulting in decreased corticospinal excitability.
Motor inhibitory processes can also be conceptualized as a process of motor response inhibition of action restraint or action cancellation in response to an external cue as measured using go/no-go tasks or the stop signal task. Voon et al.
36 showed impaired motor response inhibition as measured using a go/no-go task in 30 patients with motor functional with mixed positive movements (e.g., tremor, dystonia, myoclonus, and gait abnormalities, rather than paralysis)with otherwise intact general cognitive functioning. In the go/no-go task, participants responded as quickly as possible to the go signal (any letter) and were required to withhold responding to an infrequent stop signal (the letter X). The authors suggest that the expression of abnormal and excessive movement may in part reflect a failure of inhibition. Motor inhibition and intention have been conceptualized as two sides of the same coin, with nonconscious motor inhibition
37 and intentional and cued motor inhibition
38–40 implicating the supplementary motor complex (the same region also implicated in motor intention).
However, a study using a motor inhibitory go/no-go functional magnetic resonance imaging (fMRI) task did not support either abnormalities in intention or inhibition. Cojan et al.
41 compared a patient with functional paralysis with 30 healthy volunteers using a go/no-go task. The finding that the contralateral motor cortex had decreased activity suggested a primary impairment in execution. In this patient, the right inferior frontal cortex (IFC), a region implicated in motor response inhibition, was activated by no-go trials for the unaffected hand. By contrast, patients with simulated weakness activated the right IFC during go trials for the simulated affected hand. These results suggest that feigned paralysis engages a voluntary motor inhibitory process during action restraint but functional paralysis does not.
41 The authors suggested that the study thus did not support theories implicating intention or inhibitory processes. However, given the small sample size, interpretation of the findings is limited.
Inhibitory processes have also been investigated on a local cortical level. Short latency intracortical inhibition (SICI) has been shown to be impaired in three studies in psychogenic dystonia.
42–44 SICI involves a subthreshold conditioning stimulus applied 1–5 ms before the TMS pulse, which suppresses the amplitude of the MEP, an effect suggested to be mediated via intracortical GABA
A mechanisms.
45 Espay et al.
42 showed that both psychogenic dystonia (N=10) and organic dystonia (N=8) were associated with reduced SICI and long interval intracortical inhibition, along with shorter duration of the cortical silent period. The authors suggest that similarities between both disorders might point toward abnormal cortical and spinal excitability as a consequence of the dystonia or that these represent endophenotypic abnormalities.
42 To extend these findings, Avanzino et al.
43 measured inhibition of the unaffected arm of patients with fixed dystonia (N=12, of which four had complex regional pain syndrome) showing reduced SICI in both hemispheres. That the unaffected arm also showed reduced SICI suggested that decreased inhibition might predispose an individual to development of dystonia. The authors also showed a shorter corticospinal period in both psychogenic and organic dystonia. Fixed dystonia is believed to be commonly of psychogenic origin.
46 Quartarone et al.
44 confirmed abnormal reduction in SICI among patients with psychogenic dystonia (N=10) and organic dystonia (N=10) compared with healthy controls. Quartarone et al.
44 also measured paired associative stimulation (PAS) to measure plasticity. PAS involves repetitive low-frequency median nerve stimulation paired with TMS over the contralateral motor cortex. Changes of PAS-induced cortical excitability have similar features to associative long-term potentiation. The authors show that organic, but not psychogenic, dystonia is associated with an increase in plasticity. Schwingenschuh et al.
47 also studied the R2 blink reflex recovery cycle among patients with atypical or psychogenic blepharospasm (N=9) and benign essential blepharospasm. The recovery index was disinhibited in nine of 10 participants with benign essential blepharospasm but not in any of the patients with atypical blepharospasm, suggesting that brainstem interneuron excitability is intact.
47Thus, some but not all evidence points toward a possible impairment in inhibitory function including possible engagement of negative motor imagery, motor response inhibition, and impaired short intracortical inhibitory processes.
Corticostriatothalamic changes.
Two important functional imaging studies have shown impairments in subcortical circuitry among patients with functional paralysis and dystonia. Vuilleumier et al.
48 showed that patients with unilateral sensorimotor loss (N=7) had decreased contralateral thalamic and basal ganglia activity to passive vibration using SPECT (
Figure 2).
48 Caudate hypoactivity was associated with poor recovery on follow-up. The authors emphasize potential impairments in striatothalamocortical circuits, highlighting the caudate as a potential region allowing interaction between limbic and motor circuitry. Schrag et al.
49 studied patients with unilateral psychogenic dystonia (N=6), patients with organic dystonia (DYT1; N=5), and healthy volunteers (N=6) comparing participants at rest, fixed posture, and paced ankle movements with PET, carefully controlled for movement. Across all tasks, psychogenic dystonia was associated with greater basal ganglia and cerebellar and decreased primary motor cortical blood flow, compared with healthy volunteers and patients with organic dystonia (
Figure 2). Both patients with organic dystonia and psychogenic dystonia had greater right DLPFC activation during movement compared with rest. By comparing psychogenic and organic dystonia, the authors suggest that whereas abnormal prefrontal activity may be common to both disorders, abnormal basal ganglia activity may be specific to psychogenic dystonia. These studies suggest potential abnormalities in subcortical function; differences in the direction of basal ganglia activity in these two studies may be a function of the symptom presentation (paralysis versus dystonia) or task (passive vibration versus movement).
Anatomical differences have also been investigated in functional paralysis. Aybek et al.
50 showed that patients with motor FND (N=15), compared with healthy volunteers (N=25), had greater cortical thickness in bilateral premotor regions with nonsignificant correlations with symptom severity and illness duration. Nicholson et al.
51 further showed that patients with motor FND (N=14), compared with healthy volunteers (N=31), had decreased left thalamic volumes in region of interest analyses.
Sensory and Visual Function
Evoked potentials, event-related potentials, and rCBF in FNDs.
Techniques.
Repeated peripheral stimulation of any sensory modality produces a series of corresponding electrical evoked potentials (EPs) recordable at various levels of the ascending sensory tracts and at the cortex (
Table 3). Positive and negative components comprising the EP correspond roughly to successive stages of information processing of the ascending sensory stimulus. Lesions affecting sensory pathways generally reduce the amplitude of EP components or increase their latency.
A related technique, the oddball task, assesses higher-order cognitive processing of the stimulus information. In the oddball task, the patient responds to one type of stimulus (rare targets) while ignoring another type (common nontargets). This yields both a sensory EP, as described above, and an additional late component termed the P300. The P300 is a cognitive component in the sense that it occurs only in response to rare target stimuli, and the P300 is relatively independent of the physical characteristics of the stimulus but is critically dependent on cognitive factors such as the patient’s attention and expectation. It is termed an event-related potential (ERP) to distinguish it from the purely sensory EP.
Whereas EPs and ERPs represent the most direct measurements of sensory information processing, rCBF offers an indirect assessment of the functional state of the sensory cortex and other areas of the brain. A recent trend has been to measure both electrical and hemodynamic signals among patients with FNDs in the same study.
Somatosensory system.
The somatosensory evoked potential (SEP) has long been used for diagnostic purposes to assess the functional integrity of somatosensory pathways in FND sensory loss. A seminal investigation by Hernandez-Peon et al.
52 assessed a 15-year-old girl manifesting left-side glove and stocking anesthesia. Stimulation of her normal right forearm produced the expected SEP over the left parietal somatosensory area, but stimulation of her numb left forearm produced no definite response over the right somatosensory area. Because Hernandez-Peon et al.
53 had previously discovered in cats that stimulation of one sensory modality could suppress EPs in another sensory modality, they invoked an attentional mechanism to explain their findings from a patient with FND. Similar reports soon followed of reduced or absent SEPs over the somatosensory cortex corresponding to the affected side of patients with FND with unilateral
54,55 or bilateral
56 anesthesia, and it is commonly found that the diminished SEPs returned to normal after remission of clinical symptoms.
An important aspect of this work was the finding that SEP reduction was seen with stimulation of the skin and with low-intensity stimuli near the perceptual threshold, but not with stimulation of the ulnar nerve, or with higher-intensity stimuli, suggesting that inhibitory corticofugal feedback suppressed the initial somatosensory transduction at the receptor level. Explanatory concepts of attentional dysfunction and inhibitory corticofugal feedback were soon combined.
57 A patient’s inability to attend to the area of afferent inhibition was seen as underlying the
belle indifference traditionally associated with FND. Reviewing this and other evidence, Miller
58 suggested that the anatomic location of the defective attentional mechanism was the brainstem reticular formation, but that higher cortical areas may be involved. More specifically, the right parietal lobe (particularly the secondary somatosensory area SII) was implicated in a manner analogous to the well-known neurological syndrome of left-side attentional hemineglect after right parietal lesions.
Corresponding to the diminished or absent EP, more recent work using fMRI or SPECT to measure rCBF has shown reduced perfusion of somatosensory areas corresponding to the reported sensory loss.
59 Such findings suggest that whatever the pathophysiology of FND, it can manifest at the level of the somatosensory cortex and may involve reductions in both perfusion and electrophysiological signals. Whether such somatosensory loss involves inhibition as far down the afferent chain as the receptor level, as suggested originally by Hernandez-Peon et al.,
52 has not been well studied. In addition to primary sensory areas, decreased blood flow is seen in the thalamus, anterior cingulate cortex, inferior frontal cortices, and posterior parietal cortices,
60 suggesting decreased activation of areas involved in higher-order stimulus processing and emotional regulation.
However, published results have not been uniform; several authors have found little or no evidence of SEP abnormality at the level of the primary sensory cortex
61–65 even though the patients denied any conscious perception of the stimulation. In principle, paralleling the phenomenon of cortical blindness, normal SEP results from the primary sensory cortex could have been produced in spite of lesions of a higher-order somatosensory association area producing a sensory agnosia.
61Consistent with electrophysiological findings of a normally functioning somatosensory cortex, normal somatosensory blood flow has been reported. Although there were no perfusion abnormalities in the somatosensory cortex,
48 decreased blood flow was seen in the thalamus and basal ganglia contralateral to the sensory loss, which normalized after clinical recovery. These findings suggest that sensory pathways may be functioning normally at the primary cortical level but that there may be an abnormality of top-down suppression, perhaps acting at the thalamic level. This view is strengthened by recent fMRI findings
66 of normal or nearly normal hemodynamic responses of both contralateral and ipsilateral somatosensory areas during stimulation of numb and sensate areas. However, stimulation of numb areas additionally produced specific activation of brain regions implicated in sensory integration and emotional processing, including paralimbic cortices, temporoparietal junctions, and the dorsolateral and orbital prefrontal cortex, caudate, and ventral anterior thalamus. It is difficult to reconcile the increased hemodynamic response of these areas with the decreased response seen in similar areas by earlier investigators, but both findings point to abnormal functioning of higher-order cortical association areas after normal initial processing of the stimulus.
To investigate higher-order somatosensory mechanisms in FND, a variant of the SEP technique was used by Lorenz et al.
62 A patient manifesting right forearm anesthesia was tested with innocuous median nerve stimulation, yielding normal SEP components from both the affected and unaffected limbs. SEP testing using noxious stimulation of the hands also evoked normal long latency vertex components. The authors then utilized an oddball ERP paradigm to assess cognitive functions related to stimulus processing. Again, SEP results were normal on both sides, but the expected P300 was seen only after stimulation of the normal limb. The finding that stimulation of the numb limb produced normal early sensory SEP components but failed to produce the expected later cognitive P300 ERP component strongly suggests that the blockage or suppression of sensory information can occur centrally, rather than peripherally, and at a stage after the reception and initial processing of the stimulus information at the cortical level. The authors characterize this as a cognitive deficit, consistent with the attentional explanation offered by Ludwig.
57 Another interesting aspect of the study is the authors’ use of a healthy “malingering” control who consciously attempted to feign complete numbness of the right forearm and hand. The control participant yielded the expected pattern of normal results in all testing and specifically was unable to suppress the P300 from stimulation of the “affected” limb. Although this is essentially a descriptive, qualitative case study utilizing unclear normative data, the findings are intriguing in that they suggest that sensory information in FND is available but unused for higher cognitive processing.
Morgante et al.
67 show that both patients with fixed dystonia and patients with primary dystonia are impaired at temporal discrimination, suggesting impairments of somatosensory inputs. The temporal discrimination threshold was assessed by delivering non-noxious electric shocks at a gradually increasing interstimulus interval from 0 to 400 ms until recognized as asynchronous. However, Katschnig et al.
68 compared individuals with fixed dystonia versus those with mobile dystonia and did not show abnormalities in temporal discrimination. Thus, both studies did not show any differences between functional and primary dystonia, but one study showed an impairment in temporal discrimination across both groups that was not replicated by the second study.
Visual system.
The investigation of FND in the visual modality using EP, ERP, and rCBF techniques is less commonly reported in the literature, possibly because of the relative rarity of this condition. During diagnostic testing, the presence of normal pupillary reflexes and preserved optokinetic nystagmus suggest grossly intact subcortical and cortical visual pathways and therefore a functional origin for the reported blindness. However, electrophysiological testing and structural neuroimaging are often needed to confirm the diagnosis of FND blindness by ruling out cortical pathology, including cortical blindness.
69 The pattern reversal visual evoked potential (VEP) assesses the initial response of the primary visual cortex to a reversing checkerboard stimulus. Increased latency or decreased amplitude implies dysfunction of the ascending visual pathway or of the primary visual cortex itself. In a patient complaining of severe monocular or binocular limitations, normal VEP results coupled with normal results on a neuro-ophthalmic examination are strongly suggestive of FND.
70 However, the patient with FND may be able to suppress or distort the VEP to mimic a neurologic disorder; therefore, close observation during testing is necessary. Another limitation of the VEP is that it derives mainly from the primary visual cortex (Brodmann’s area 17) and does not reflect the integrity of higher visual association areas. Normal VEPs may be recorded even in neurological patients suffering from large lesions in areas 18 and 19 manifesting as cortical blindness.
71,72A case study of FND blindness
73 using VEPs and fMRI revealed the expected pattern of smaller VEP amplitudes over blind visual quadrants compared with intact quadrants (which normalized after successful psychotherapy). However, the fMRI was normal in both blind and intact quadrants. Because VEPs from primary visual cortex may be modulated by attentional focus, the authors suggested a mechanistic role for attention consistent with the somatosensory FND literature. In sharp contrast, the converse pattern of normal VEPs but reduced fMRI blood flow activation in the primary visual cortex has been reported in FND blindness.
74 An accompanying increased blood flow activation of the posterior cingulate cortex, insula, temporal poles, thalamus, and striatum, with decreased hemodynamic activation in the anterior cingulate cortex, suggested inhibitory modulation of the primary visual cortex through increased activity of limbic areas with a shift toward implicit (nonconscious) visual processing. In an FND case study, Okuyama et al.
75 similarly found that decreased visual acuity and blurred vision were accompanied by normal perfusion of the primary visual cortex but that visual association areas had decreased regional blood flow.
Perhaps a better way to assess attention or other cognitive factors in FND independent of peripheral distortion is to measure the P300. In an early study of patients with functional blindness, a P300 was still elicited by unreported visual stimuli but with smaller amplitude than normal.
76 The presence of a P300 confirms that visual information reached the cortex, that the attended target and ignored nontarget visual stimuli could be differentiated, and that the relative probability of each could be used to form expectations. The finding of an abnormally small P300 is consistent with an attentional impairment.
77In summary, EP, ERP, and rCBF studies of patients with sensory FND sometimes show deficits at the level of the primary sensory cortex but more reliably show abnormalities in a variable constellation of higher-order association areas and in some nonsensory subcortical structures. The most illuminating studies are those involving ERP recording. In both the somatosensory and visual systems, patients with FND show normal sensory EPs but reduced or absent cognitive ERPs in response to stimulation of the affected area of the skin or visual field. This finding itself is strongly suggestive of an abnormally functioning attentional mechanism active across sensory modalities. Hemodynamic studies have been only sporadically congruent with EP findings in primary sensory cortices and have not yielded a consistent picture regarding the higher-order association areas thought to underlie the cognitive P300 findings. Clearly what is needed to resolve the divergent findings is a series of ERP studies that incorporate hemodynamic as well as electrophysiological measures and involve a reasonable sample size of clinically similar patients. Such a design would allow evaluation of both sensory and cognitive aspects of FND in a single study.
Also needed are studies of stimulus mode and intensity. Ghaffar et al.
78 investigated unilateral FND sensory loss and found that contralateral SI was not activated by unilateral stimulation but was activated by bilateral stimulation. They suggested that inconsistencies in the literature may be related to whether unilateral or bilateral stimulation was applied. Inconsistencies might also be related to stimulus intensity. Low intensities appear more likely to reveal abnormalities.
54,79 Or they might be attributable in part to the level at which the somatosensory system is stimulated. Earlier work found SEP abnormalities only when the skin receptors themselves were stimulated; stimulation of the ascending sensory (ulnar or tibial) nerve yielded normal SEPs
61 in the same patients.
79 Differences in the patient’s presenting symptoms (anesthesia versus paresthesia, blindness versus acuity loss, or pure sensory versus sensorimotor) may also be an important area for future studies.
PNES.
Interictal EEG.
Several studies have shown nonspecific interictal EEG abnormalities among patients with PNES. Reuber et al.
80 show that 53.8% of patients with PNES only (N=130) had nonspecific abnormal interictal EEGs, which is 1.8 times greater than healthy controls. Reuber et al.
81 also show that 22.3% of patients with PNES (N=206) had an abnormality of nonspecific interictal EEGs, MRI changes, or neuropsychological deficits. Similarly, LaFrance et al.
82 showed that 11 of 38 patients (29%) with PNES had interictal EEG abnormalities and 16 of 38 (42%) had abnormalities on MRI, despite the absence of a “focal neurological lesion” associated with their PNES. These EEG findings are nonspecific and do not indicate evidence of epilepsy.
Heart rate measures.
The concurrent recording of the ECG during a video EEG has been studied for the potential for additional diagnostic information, aiding in distinguishing PNES from epilepsy and from physiologic nonepileptic events. A very rapid increase of the heart rate at seizure onset (especially in the absence of motor features explaining tachycardia) is suggestive of an epileptic etiology. The ictal heart rate is higher and the ictal heart rate increase is more rapid in epilepsy than in PNES.
83,84 Interictal heart rate variability parameters show abnormalities among patients with PNES but do not help to distinguish between patients with PNES and those with epilepsy.
85,86 Unlike that seen in epileptic seizures, the heart rise increase in PNES is usually commensurate with the physical activity involved in the seizure,
87 suggesting differences in mechanisms underlying the change in heart rate.
Prolactin.
Serologic measures have been used to differentiate epilepsy from PNES, the most useful being prolactin (PRL). Elevated serum PRL among patients with generalized epileptic seizures (ES) helped distinguish epilepsy from PNES.
88 Many studies have since been conducted measuring PRL among patients with PNES, finding that when PRL did not rise postictally, the average sensitivity to PNES was 89% across the studies.
89 Furthermore, studies of PRL in ES versus PNES have since shown that serum levels are elevated on average in 88% of generalized tonic clonic (GTC) epileptic seizures, in 64% of temporal lobe complex partial epileptic seizures (CPSs), and in 12% of simple partial epileptic seizures. False positives for epilepsy include treatment with dopamine antagonists and some tricyclic antidepressants, breast stimulation, and syncope. False negatives occur with use of a dopamine agonist, or with status epilepticus, because PRL has a short half-life and may attenuate in postictal release.
90 PRL may also fail to rise after frontal lobe epileptic seizures. The American Academy of Neurology Therapeutics and Technology Assessment Subcommittee published a report on the use of serum PRL in differentiating ES from PNES. The review of the PRL seizure literature concluded that a twice normal relative or absolute serum PRL rise, drawn 10–20 minutes after the onset of the ictus, compared against a baseline nonictal PRL, is a useful adjunct in the differentiation of GTC epilepsy or CPS epilepsy from PNES.
91Other serum measures.
Other serum measure studies to differentiate GTC ES from PNES have included the use of elevations in peripheral white blood count,
92 cortisol,
93 creatine kinase,
94 and neuron-specific enolase
95; however, there was limited discriminative power of these serological tests in differentiating epilepsy from PNES.
96 Capillary oxygen saturation on pulse oximetry is lower for epilepsy than for PNES.
97Brain-derived neurotrophic factor (BDNF) levels have been shown to be lower among patients with PNES than healthy controls but did not differ from patients with epilepsy.
98 The similarities between ES and PNES were thought to be attributable to the stress of the seizure, despite different underlying pathophysiologies. Similarly, Deveci et al.
99 analyzed serum BDNF levels among patients with FND, showing low BDNF levels in both patients with FND and in those with depression compared with healthy volunteers.
Neural networks and anatomical differences.
Abnormalities in connectivity and neural networks in PNES have been investigated using EEG and resting state fMRI. Knyazeva et al.
100 studied PNES (N=13) and healthy volunteers (N=13) using multivariate phase synchronization in interictal high-density EEG and did not show any group differences; however, their results show decreased prefrontal and parietal synchronization with a greater number of PNES events. Barzegaran et al.
101 further analyzed these results using graph theory, showing that patients with PNES had similar local and global connectivity and small-world structure, and the deficits in local connectivity correlated with the number of PNES per month.
Using resting-state fMRI, van der Kruijs et al.
102 showed that patients with PNES (N=11) compared with healthy controls (N=12) had greater functional correlation between regions implicated in emotion and self-perception (insular) and motor preparation (precentral and central sulcus) and that dissociation scores were positively correlated with this connectivity. Ding et al.
103 used graph theoretical analysis to compare patients with PNES (N=17) and healthy volunteers (N=20) applied to both functional (resting state fMRI) and anatomical (diffusion tractography) networks. Subjects with PNES had abnormal small-worldness with more regular (lattice-like) organization of large-scale functional and structural networks, indicative of impaired global integration and less information propagation. The coupling strength or relationship between functional and structural connectivity was decreased in patients with PNES compared with healthy controls with high specificity (75%) and sensitivity (77%) to differentiate groups. Other diffusion tensor imaging (DTI) studies found that patients with PNES (N=16) had significantly higher DTI indices compared with 16 age- and sex-matched healthy controls in the left corona radiate, left internal and external capsules, left superior temporal gyrus, and left uncinated fasciculus, areas associated with emotional regulation and motor pathways.
104In contrast with findings in functional paralysis with greater bilateral premotor cortical thickness,
50 Labate et al.
105 also showed that patients with PNES (N=20), compared with healthy volunteers (N=40), had decreased volume and cortical thickness in the right premotor and motor cortex and bilateral cerebellar regions.
Self-Monitoring or Attention to Self
Attention has been proposed to be important in the development and maintenance of medically unexplained symptoms. Brown suggests that “all somatoform conditions with the exception of those involving observable physical phenomena are governed by the same basic mechanism, namely the repetitive reallocation of high-level attention on to symptoms.”
106 The issue of attention to self can be observed on a clinical level and has been used to support clinical diagnosis. For instance, functional tremor has been shown to be sensitive to distractibility, including by performance of a voluntary rhythmic movement,
107 a ballistic movement,
108 an auditory rhythm,
109 or mental concentration on serial sevens.
110 van Poppelen et al.
111 measured visual attention directed to the affected limb in videos of functional tremor (N=13) and other tremor disorders (N=17) such as Parkinson’s or essential tremor, showing that functional tremor was associated with attention focused on the affected limb 66% of time compared with 32% in controls. Attention focused on a novel voluntary movement is required for optimal motor function, suggesting the movement to be voluntary and possibly novel (rather than overlearned) in nature. Studies also suggest that attention plays a role in the underlying pathophysiology of FND. Kumru et al.
112 compared healthy volunteers with patients with functional tremor (N=6), Parkinson’s disease (N=9), and essential tremor (N=11), using a prolonged simple reaction time task (to hit a switch 15 cm away) to a visual signal during contralateral hand tremor versus at rest. In the tremor condition, patients with functional tremor had prolonged reaction time compared with individuals with essential tremor. Both patients with functional tremor and healthy volunteers had prolonged reaction times in the tremor compared with rest conditions, whereas no differences were observed in the other two groups. These findings are suggestive of an interference during dual task performance, which results from a bottleneck in central processing of attention, suggesting that only one task can be processed at a time. Similarly, in healthy volunteers, phasic voluntary movement interferes by prolonging reaction time with a dual task mediated by a nonselective inhibitory influence (suppression of MEPs) exerted on the ipsilateral motor cortex.
113ERP, TMS, and fMRI studies have also addressed this issue of self-monitoring. In subjects with functional paralysis (N=6), Roelofs et al.
114 also showed that during the preresponse phase of the response conflict Flankers task, N2 congruency (reflecting preresponse conflict) increased for the affected hand compared with the unaffected hand. There was no difference in the error-related negativity, reflecting postresponse error monitoring. The authors suggest that this reflects greater anterior cingulate hyperactivity and self-monitoring during movement initiation but not execution or feedback.
114Neural regions involved in self-monitoring, or the default mode network, have also been implicated in FND. As discussed above, implicitly induced motor imagery of the affected hand recruited VMPFC and superior temporal cortices compared with the unaffected hand, suggesting heightened self-monitoring.
18Regions implicated in self-monitoring have also been implicated in FC with motor networks. Cojan et al.
41 in their comparison of one patient with functional paralysis and 30 healthy volunteers using the go/no-go task also showed greater FC between the VMPFC, precuneus, and posterior cingulate cortex with right M1, suggesting a role for self-related representations or emotions in influencing voluntary motor activity (
Figure 2). These findings are contrasted with that of de Lange et al.,
22 who did not show any connectivity between VMPFC and sensorimotor regions in a comparison of the affected versus unaffected sides using self-related motor imagery.
This enhancement in attention to self or self-monitoring has been proposed to play a role in increasing the precision of priors, expectations and beliefs, as further discussed below.
Cognition
Early studies by Flor-Henry et al.
115 showed that FND was associated with bifrontal impairment and dysfunction of the nondominant hemisphere in a small study of patients with “hysteria” (N=10) compared with matched controls. Studies by Kalogjera-Sackellares and Sackellares,
116 Sackellares et al.,
117 Wilkus et al.,
118,119 Strutt et al.,
120 and Binder et al.
121 focusing on PNES compared with mixed PNES and epilepsy or ES alone showed neuropsychological impairment in multiple domains, with PNES function shown to be similarly or less impaired compared with mixed presentations or ES. For instance, Kalogjera-Sackellares and Sackellares
116 assessed patients with PNES (N=44; full-scale IQ=95) and mixed PNES and ES (N=9), showing a high proportion of low average to borderline (41.5%) impairments in the Halstead-Reitan Neuropsychological Battery in both groups in multiple domains (mental flexibility, problem solving, spatial memory, auditory perception and discrimination, and motor speed and coordination). The Halsted Impairment Index (overall level of performance) was impaired in 63% of participants.
116 The authors suggest that the results may also be related to a high incidence of traumatic brain injury (58%) and history of substance use (17%). Strutt et al.
120 addressed this potential confounder comparing patients with PNES (N=33, IQ=90.2, SD=9.63) and temporal lobe epilepsy (N=25) using stringent exclusion criteria, including recent substance use disorder, history of head trauma, use of psychoactive medications, and other major psychiatric disorders. They show that individuals with PNES have greater impairments in attention and working memory (digit span, spatial span, working memory index), whereas patients with ES had greater impairments in verbal and visual memory. Compared with normative scores, the PNES group means for attention, working memory, information processing, language, and set shifting were below average. The test for effort (Test of Memory Malingering [TOMM]) was unremarkable and within normal limits. The authors suggest that observations of more generalized impairments may be a function of attention or working memory deficits. Binder et al.
121 have also shown that neuropsychological impairment is associated with emotional factors in the PNES population but not the population with epileptic seizures.
Several studies have focused on the question of variable effort using symptom validity tests. Symptom validity test measures are designed to appear difficult but are actually very easy, such that individuals with known impairments such as moderate to severe traumatic brain injury or mental handicaps respond correctly more than 90% of the time.
122 Binder et al.
123 compared individuals with intractable seizures, showing that patients with PNES (N=34) had fewer correct on the Portland Digit Recognition Test (PDRT). Drane et al.
124 compared individuals with PNES (N=43) and ES (N=70) using the WMT and a battery of cognitive tests, which provided the Dodrill Discrimination Index (DDI) or percentage of scores falling in the abnormal range. Although both groups had the same DDI score (50.3 versus 52.6), patients with PNES who failed the WMT performed much worse on the DDI than those who did not fail the WMT. Similarly, Locke et al.
125 showed that performance on the TOMM had a better correlation with cognitive outcomes than did neuropathology or psychopathology. However, Cragar et al.
126 used four different symptom validity tests including the PDRT, Digit Memory Test, Letter Memory Test, and TOMM, showing similar poor effort in PNES (N=21; 24%), ES (N=41; 22%), and PNES plus ES (N=18; 11%); these results suggest that these measures would not be useful in discriminating clinical diagnostic categories. Williamson et al.
127 showed that failure on the WMT among patients with PNES (N=90; 35%) was associated with a history of physical or emotional abuse (reported as yes or no to the different abuse types) and was not associated with obvious financial incentive. The authors suggest that abuse may be associated with dissociative tendencies, which might interfere with encoding novel information or verbal learning.
In contrast with the studies focusing on PNES, Voon et al.
36 conducted a study focusing on PMD (N=30), which excluded patients with traumatic brain injury and controlled for depression and anxiety, Voon et al.
36 did not show any differences from healthy volunteers in the domains of attention, working memory, processing speed, planning, spatial orientation, and response conflict. Similarly, Heintz et al.
128 show that despite subjective reports of greater cognitive complaints, patients with psychogenic jerky movement (N=26) did not show any differences from healthy volunteers on neuropsychological tests but showed noncredible test performance on a symptom validity test. Verbal learning was correlated with symptom validity test results and severity of depression and anxiety.
Thus, patients with PMD appear to have intact neuropsychological performance, whereas patients with PNES appear to have impairments similar to those with ES. These findings may reflect differences in phenomenological presentations between PMD and PNES or may reflect differences in the association with abuse history, the relationship with effort, and the presence of anxiety or depression.
Expectation
Edwards et al.
129 elegantly describe a testable model focusing on expectation and attentional modulation. The primary failure is described as an aberrant “prior” expectation, or a percept or belief (e.g., sensory percept or modulation of movement) that is held with undue certainty due to top-down attentional modulation of synaptic gain (postsynaptic responsiveness). The authors describe a secondary failure of inference in which the actual somatosensory percept is attributed as a symptom to explain why the prediction is abnormal. This model highlights impairments in expectation including the influences of experience and culture as well as impairments in attention and information processing.
The improvement of symptoms in response to suggestibility or placebo is often used in supporting the FND diagnosis and supports the role of expectation. Edwards et al. have shown enhanced responding to placebo among patients with fixed dystonia with an immediate response of the dystonic symptom to botulinum toxin consistent with a placebo response.
130 Kenney et al.
110 have also shown that patients with psychogenic tremor (N=12) were more susceptible to suggestibility compared with individuals with essential tremor (N=33). Patients were told that “tremor has been shown to improve when a vibrating source is applied to the body” before a vibrating tuning fork was applied to their forehead.
110 The subjective experience of tremor has also been shown to differ from organic movement disorders. Pareés et al.
131 showed that patients with PMD (N=8) reported more subjective tremor in the waking day compared with those with organic tremor, despite having very little tremor recorded by objective actigraphy. The authors suggest that the subjective experience might be reflective of the patient’s expectation.
Higher-order cognitive processes and decision making may also play a role in information processing of somatic symptoms. Using a probabilistic reasoning task, Pareés et al.
132 show that that patients with PMD (N=18) requested less information before making a decision, suggesting possible abnormal inferences when making decisions about somatic symptoms.
Voluntariness or Agency
The symptoms of FND are experienced as involuntary or not under the person’s control, although the functional movement itself might utilize voluntary pathways. The phenomenon of entrainment, in which a functional tremor entrains to the frequency of a repetitive voluntary movement, is suggested to be a result of sharing of the same central oscillator.
133 Entrainment has been shown to have low sensitivity but 100% specificity in the diagnosis of functional tremor.
134Several other studies support similarities between voluntary actions and functional symptoms. The Bereitschaftpotential (BP), or premovement potential, is a slow negative EEG shift occurring before onset of a voluntary movement and is a characteristic of a voluntary movement. Terada et al.
135 first showed that psychogenic myoclonus is commonly, although not always, associated with a preceding BP. Demonstration of a BP preceding myoclonus is a gold standard sign that the myoclonic symptom is psychogenic in nature, but the lack of a preceding BP does not rule out a psychogenic diagnosis.
133 Maruff and Velakoulis
136 compared a patient with functional paralysis with healthy volunteers and a patient with an actual arm injury using a visually guided pointing task to examine speed-accuracy trade off. The patient touched the end of a vertical line, then a target box with differing target sizes, five times, as quickly and as accurately as possible. The duration of this sequential movement was recorded. Healthy volunteers performed real and imagined movements made while feigning injury or not. The patient with functional paralysis performed both real and imagined movements with either the affected or unaffected arm. Among healthy individuals, the patient with an actual arm injury, and the unaffected arm of the patient with functional paralysis, real and imagined performance had similar duration and conformed to Fitt’s law (or the physiological process governing the tradeoff between speed and accuracy as target size is varied). However, in functional paralysis of the affected arm and healthy volunteers feigning paralysis, real performance was slow and did not conform to Fitt’s law, whereas imagined performance conformed to Fitt’s law.
136 These findings suggest similarities between functional paralysis and feigned paralysis in the inability to interfere with environmental constraints on speed accuracy relationships for imagined movement.
Some evidence also exists for possible impairments in voluntary motor function in functional disorder. Sackellares and Sackellares
137 showed decreased motor speed (finger tapping) and grip strength and decreased intermanual performance asymmetry among patients with PNES (N=40) compared with healthy volunteers. Similarly, Kenney et al.
110 compared patients with functional tremor (N=12) and essential tremor (N=33) and showed greater impairments in finger tapping among the former.
The question arises as to why the symptom should be experienced as involuntary when it utilizes voluntary pathways. This question was indirectly addressed by Voon et al.,
138 who compared functional tremor with voluntary mimicked tremor in a within-subject design (N=8) and showed decreased activity in the temporoparietal junction (TPJ). Functional tremor compared with voluntary movement was also associated with lower FC between the right TPJ and regions involved in sensory feedback (sensorimotor cortices and cerebellar vermis) and limbic regions (ventral anterior cingulate and ventral striatum).
138 Contemporary motor theory proposes that motor control follows a feed-forward model in which self-generated movements are accompanied by a sensory prediction of the motor outcome. The movement prediction usually matches the sensory outcome giving rise to a sense of self-agency. A mismatch may thus give rise to the sensation that we are not in control of our movements. The right TPJ has been proposed by Decety and Lamm
139 to act as a comparator of internal predictions and actual external events and may account for cognitive processes such as agency, theory of mind, and attention. Because sensory feedback in the functional tremor study appeared to be intact in both conditions, the decrease in TPJ activity was proposed to represent an abnormality in internal prediction. This would then lead to a mismatch of prediction and outcome, decreased activity in the comparator TPJ function, and the experience that the movement is not under the individual’s control. Whether these findings are specific to psychogenic disorders or can also be observed in organic movement disorders remains to be established.
Kranick et al.
140 further assessed an implicit measure of agency during voluntary movements in functional movement disorders using an action-binding task. Participants viewed a rotating clock and made judgments of when actions and outcomes occurred in three conditions: an action alone (button press), an outcome alone (tone), or an action-outcome pairing (button press − tone). In healthy volunteers, during the action-outcome pairing, the action judgment occurs much later in time, and the outcome judgment occurs much earlier in time. This action-outcome binding is believed to underlie our subjective sensation that our actions are associated with an effect. Kranick et al. showed that patients with FND had a decrease in action binding, consistent with their decreased sense of agency. Action-outcome binding,
141 along with attention to intention,
25 and the urge to move
27 have been associated with the supplementary motor complex in both fMRI and single-unit recordings. These findings are consistent with abnormal SMC activity during voluntary motor preparation in functional movement.
23Pareés et al.
142 also showed that patients with FND with movement symptoms have impaired sensory attenuation compared with healthy controls. Sensory attenuation describes the reduction of the intensity of sensation caused by movements that are self-generated compared with by others. This phenomenon is believed to be important in the labeling of movements as self-generated and has been associated with a loss of agency for movement. Sensory attenuation can be assessed using the force matching paradigm in which individuals are asked to match a force delivered to their finger either by pressing directly on their own finger with the other hand (self) or by operating a joystick that causes a robot arm to press on their finger (other). Whereas healthy controls overestimate the self-generated force, there were no differences in the estimation of force between self- and other-generated movements in the FND group.
Dissociation and Hypnosis
Dissociation.
Functional disorder in
ICD-10 is categorized under disorders of dissociation. Dissociation is defined in
DSM-IV as a “disruption of the usually integrated functions of consciousness, memory, identity or perception of the environment.” This article is not intended to provide a thorough review of the concept of dissociation (reviewed in Bell et al.
143 and Holmes et al.
144,145) and does not extensively discuss the relationship with hypnosis (reviewed in Bell et al.
143 and Hallett et al.
146). Some argue that dissociation and functional operate under similar processes, and others argue that they are separate and independent. The above-noted reviews note that evidence from a neurophysiological perspective is limited to support these psychological theories, and systematic neuropsychiatric studies are required to address these concepts from an integrated biopsychosocial approach.
Dissociation is commonly dichotomized into two forms: functional and dissociation. Cardena
147 classifies FND within a category 1 form of dissociative phenomena, characterized by an apparent dysfunction in perception, memory, or action that (a) cannot be reversed by an act of will, (b) occurs in the presence of preserved functioning of the apparently disrupted system, and (c) is reversible compared with category 2 dissociation, which includes depersonalization and derealization. Similarly, Brown
145 differentiates between the concepts of detachment and compartmentalization. Detachment describes an altered state of consciousness characterized by a sense of separation from experience including a sense of self (depersonalization), the external world (derealization), and the individual’s own body (out-of-body experiences). Functional disorders and somatoform disorders are considered disorders of compartmentalization characterized by impairment in the ability to deliberately control processes or actions that would normally be amenable to such volitional control. The compartmentalized processes continue to operate normally and are able to influence emotion, cognition, and action.
145Compartmentalization would thus imply abnormalities in explicit processes but intact implicit processes for which some evidence exists in FND. For instance, as discussed above, in functional blindness, Werring et al.
74 showed intact implicit visual pathways but abnormal explicit visual pathways consistent with the concept of compartmentalization. Similarly, in a case report on functional blindness, Bryant and McConkey
148 showed that the patient responds to complex visual information. Kuyk et al.
149 also showed that patients with PNES reporting amnesia during the PNES event were able to correctly recall information under hypnosis, whereas individuals with ES reporting amnesia were not able to do so, suggesting memory function to be intact. Roelofs et al.
15,16 also show impairments in explicit initiation processes but not implicit processes in FND, as discussed above.
On an imaging basis, also as noted above, van der Kruijs et al.
102 showed that patients with PNES (N=11) had greater FC in resting-state fMRI between the insular, inferior frontal, parietal, and precentral sulcus, which was positively correlated with dissociation scores as measured using the Dissociative Experiences Scale (DES). However, the DES has been suggested to measure predominantly detachment and not compartmentalization.
144Hypnosis.
This concept of compartmentalization reflects Janet’s theory that the mechanisms responsible for functional symptoms may be similar to those involved in hypnotic suggestion. Hypnosis is defined as an alteration in consciousness with heightened suggestibility and decreased awareness. Oakley
150 defines hypnosis as the “withholding of representations from entry into self-awareness…as a result of the inhibition by the central executive system with a separation of the executive system and awareness.” Similarities exist on a phenomenological level between hypnosis and functional symptoms (e.g., in the dissociation between subjective intention to move and the actual movement).
Subjects with high susceptibility are also more likely to be hypnotizable,
143 although the association between FND and hypnotizability is not clear. Two studies (total N=57) showed heightened suggestibility among individuals with FND or PNES compared with controls with affective disorders
151 or compared with patients with ES.
152 However, three studies (N=132), including a large study,
152–154 did not show differences between hypnotizability among patients with FND or PNES compared with either healthy or neurological controls. Hypnotizability was higher in a subgroup with a history of trauma, in which general psychopathology explained 67.3% of the variance and trauma explained 3.3% of the variance.
Other evidence for an overlap comes from the comparisons of imaging tasks in FND and hypnosis. Early small studies by Halligan et al.
10 and Marshall et al.
9 observed similar regions such as the anterior cingulate and OFC activated in hypnotic paralysis
10 and functional paralysis
9 during attempted movement. Ward et al.
155 expanded the study to 12 healthy volunteers comparing hypnotized paralysis in a patient with feigned paralysis, showing increases in right OFC and cerebellum and left thalamus and putamen in hypnotic paralysis; however, they did not show abnormalities in the anterior cingulate. Deeley et al.
11 performed a similar study (N=8) controlling for depth of hypnosis, showing greater anterior cingulate and SMA activity in the attempt to move during hypnotic paralysis compared with feigned paralysis; however, they did not show abnormalities in the OFC (
Figure 2). The authors suggest that the engagement of the SMA implies a role for motor intention and planning, and the anterior cingulate supports a role for inhibitory processes, although it may also represent action monitoring or response conflict processes.
By contrast, Cojan et al.
41 studied hypnotic paralysis using a similar go/no-go task, as described above, for functional paralysis, showing similarities in precuneus activity and FC between the precuneus and motor cortex (
Figure 2).
12 Cojan et al. suggest that there was no evidence of impairments in motor intention or inhibition, given intact activity in the motor cortex during preparation and lack of right prefrontal motor inhibitory processes in hypnotic paralysis. The authors propose that suggestion in both hypnosis and FND might act through self-monitoring processes to allow internal representations to guide behavior.
Arousal and Trauma
Arousal.
The relationship between arousal or stress and functional symptoms has been examined investigating physiological measures of arousal, the effects of stress induction on cognitive tasks, and the effects of arousal imagery on eyeblink and fMRI neural activity.
Lader and Sartorius
156 showed that patients with mixed active FND symptoms compared with those with anxiety disorders or healthy volunteers had greater baseline arousal levels, as measured by spontaneous fluctuation in skin resistance along with failure to habituate skin conductance to repeated auditory stimuli. Horvath et al.
157 extended these findings to show that patients with remitted mixed functional symptoms, compared with controls with “free floating anxiety,” similarly had a failure to habituate skin conductance response to repeated acoustic stimuli with normal baseline arousal responses. Bakvis et al.
158 further showed that patients with PNES have increased basal diurnal cortisol levels associated with a history of sexual trauma and lower heart rate variability at baseline, suggesting greater sympathetic activity. There were no differences in the dexamethasone suppression test or salivary amylase measures.
Cognitive function such as attention may be more vulnerable to conditions of stress. Bendefeldt et al.
159 studied hospitalized patients with FND (N=17), showing impairment under conditions of stress on vigilance attention and recent memory, greater suggestibility and greater field dependence. However, Bakvis et al.
160 examined the effects of a well-validated acute stress induction paradigm, the Trier Social Stress Test, administered to patients with PNES (N=20) compared with healthy volunteers (N=20) and did not show any effects of stress on attentional bias to a masked emotional Stroop task using angry, neutral, and happy faces. At baseline, patients with PNES had a positive attentional bias for masked angry faces, which correlated with baseline cortisol levels.
160 Individuals with a history of sexual abuse were also more likely to have elevated baseline cortisol and increased threat vigilance.
Seignourel et al.
161 showed enhanced eyeblink amplitude to both positive and negative valenced images paired with an acoustic startle reflex paradigm (affective acoustic startle reflex) among individuals with PMD (N=12) compared with healthy volunteers, suggesting aversive physiological responses to arousal stimuli. The study links arousal to a reflexive motor response. Voon et al.
162 assessed individuals with motor FND (N=16), showing greater amygdala activity to arousing facial stimuli (positive and negative) irrespective of valence compared with healthy volunteers (
Figure 2). Arousing stimuli were also associated with enhanced FC between the amygdala and SMA among patients. However, not all studies have replicated these findings. van der Kruijs et al.
102 studied individuals with PNES (N=11) using positive outdoor images with a Stroop task and did not show any differences in activation compared with healthy controls. In a single within-subject fMRI case report using a vocalization task, Bryant and Das
163 showed that after speech recovery, but not during mutism, inferior frontal gyrus activity was functional connected with anterior cingulate activity and negatively with amygdala activity. The authors suggest a potential interaction between speech networks and regions such as the anterior cingulate that regulate amygdala activity.
163Trauma.
FND (conversion disorder) was historically conceptualized as related to repression of traumatic memories. Studies in PNES have frequently documented an increased incidence of childhood trauma including sexual abuse.
164 In studies focusing on functional paralysis or PMD, a greater incidence of physical or emotional abuse has been described along with greater subjective fear associated with the abuse.
165 However, these forms of FND appear to be less likely associated with sexual abuse.
Bakvis et al.
158 have shown that a history of sexual abuse among patients with FND is associated with greater baseline cortisol levels, along with greater attentional bias toward masked angry faces.
160In a single case study in functional paralysis, Kanaan et al.
166 showed greater amygdala and right inferior frontal activity and decreased motor activity to cued recall of a clinically repressed emotional event compared with other severe personal events. In an extension of this study, Aybek et al.
167 compared stressful life events assessed using the Life Events Difficulties Schedule among patients with motor FND (N=12) compared with healthy controls (N=13), focusing on escape (based on the threat and extent to which illness might allow escape from the consequences) compared with severity (equally threatening control events). The escape events were associated with longer reaction times and were perceived as less upsetting than severe events. In the contrast of escape versus severe events, patients with FND had greater left DLPFC and decreased left hippocampal activity, along with increased right SMA and TPJ activity (
Figure 2). The authors suggest that these findings represented the Freudian concept of repression, in which memory repression was associated with greater DLPFC activity and lower hippocampal activity, with a decrease in the subjective experience of the memory but with functional symptoms associated with greater SMA activity. For both escape and severe events, patients with FND had lower right IFC activity suggesting a role for cognitive control. Patients also had greater FC between amygdala and SMA and cerebellar regions for both escape and severe events, consistent with previous findings of greater limbic-motor interaction.
162Aybek et al.
168 also looked at the responses of these 12 patients with FND (compared with controls matched for age, gender, IQ, and sexual trauma) to sad or fearful faces versus neutral expressions. Anxiety was ruled out as a contributing variable. Patients with FND had greater activity in the left amygdala in response to fearful stimuli; this activity did not diminish over time. Increased activity was also seen in bilateral premotor/SMA, left DLPFC, left cingulate cortex, and periaqueductal gray (PAG). The authors conclude that progressive sensitization to fear in the amygdala may correspond to persistent somatic responses to stress and threats, leading to enduring symptoms. Furthermore, the activation of PAG is suggestive of a similarity to animal models of “freeze response” to negative threats. The nonhabituation to fear in amygdala may be a form of biomarker for people with FND.
These findings emphasize that although psychological factors may not be necessary to the diagnosis of FND, they likely play a relevant pathophysiological role in a large proportion of patients. The issue of trauma and repression may be particularly relevant to a subset of patients and may be more prevalent among patients presenting with PNES symptoms. Trauma may not be relevant in all patients and may represent one pathway in the expression of functional symptoms. Nevertheless, these findings emphasize that although psychological factors may not be necessary to the diagnosis of FND, they likely play a relevant pathophysiological role in a large proportion of patients.
Summary of Implicated Neural Regions
The following summarizes the neural regions implicated in motor FNDs (
Figure 2). Perez et al.
169 noted that functional neuroimaging studies have been limited to date in PNES and FND; however, inspection of these activation foci suggests alterations in regions mediating emotional processing, regulation, and awareness (perigenual anterior cingulate cortex/VMPFC, insula, amygdala), cognitive control (DLPFC, dorsal anterior cingulate cortex [dACC], inferior frontal gyrus), self-referential processing (TPJ/posterior cingulate cortex/precuneus), and motor planning (SMA).
169The influence of arousal or negative events on motor function has been hypothesized in FND implicating limbic-motor interactions. For instance, greater amygdala activity has been observed with arousing stimuli in motor FND with enhanced amygdala-SMC FC observed with arousing
162 and negative events.
167 Similarly, in functional mutism, greater FC was observed between the IFC and amygdala.
163 The SMC is implicated in processes of motor initiation, intention, and urge and also in inhibitory processes. Decreased SMC activity has been shown during voluntary movement in PMD.
23 Increased SMC activity has also been observed in functional paralysis in response to traumatic escape events
167 and in attempted movement during hypnotic paralysis.
11 The representation of functional motor symptoms is also proposed to lie in the SMC and premotor cortex.
129 Because the SMC and amygdala do not have a direct anatomical connection, connectivity between motor-limbic regions may be mediated by the striatum. Striatal hypoactivity has been shown to vibration in functional paralysis
48 and hyperactivity has been shown across all movement and rest tasks specific to psychogenic dystonia and not organic dystonia.
49Enhanced self-monitoring and the influence of internal thoughts on motor function implicates the default mode network in FND. Implicit imagery of the affected compared with unaffected hand increased activation of default mode network regions, including the VMPFC and superior temporal cortex in functional paralysis, suggests a role for enhanced self-monitoring.
18 Greater VMPFC activity was observed during a tremor-inducing motor task in functional tremor but not in essential tremor.
21 The default mode network including the VMPFC and precuneus has increased FC with motor cortex in functional paralysis, suggesting an influence of internal thoughts on motor function.
41The DLPFC has also been implicated in intention, attention, and trauma suppression in FND. Impaired activity in the DLPFC has been shown during attempted movement in functional paralysis.
8,13,14 Decreased DLPFC-SMA FC during intentional movement in positive functional motor symptoms
23 and, conversely, increased connectivity during imagery in functional paralysis
22 has been observed. The DLPFC is further activated in the context of possible suppression of traumatic escape events.
167 However, DLPFC activity may not be specific to FND and can also be observed with attempted movement in DYT1 dystonia.
49The dorsal anterior cingulate cortex (dACC) has been shown to be hyperactive during attempted movement in functional paralysis
9,14 and in hypnotic paralysis.
11 The dACC activity has been postulated to be involved in inhibitory processes, action monitoring and response conflict and is also implicated in the context of negative outcomes, reward expectation and prediction error. Furthermore, functional tremor compared with voluntary mimicked tremor was associated with lower TPJ activity, which was suggested to be related to mismatch in the comparison of predicted and actual sensory outcomes.
138 Similarly, decreased supramarginal gyrus activity of attempted versus feigned paralysis suggests impairments in the integration of body schemes and environmental cues.
14The insula, which is involved in representation and subjective awareness of interoceptive or bodily signals, has also been shown to be implicated in FND. Increased BOLD activity has been reported in FND during attempted movement of a paralyzed limb (left),
13 motor preparation of voluntary action (left),
23 an escape-severe traumatic condition (right),
167 and increased rCBF during rest (left).
21 Decreased insular activity has been reported with motor imagery.
14Summary and Recommendations
The findings presented in this review suggest a model for understanding FND as resulting from a mix of higher-order influences (e.g., attention to self or expectation) and bottom-up limbic influences (e.g., trauma and arousal) interacting with and influencing basic motor function (e.g., intention, inhibition), implicating complex associative regions and processing upstream of primary motor and sensory cortices. Such a model might suggest some underlying mechanistic similarities among differing neurological presentations and possible similarities with other functional medical syndromes. The pattern of higher- and lower-order influences interacting with basic neurological processes (motor, sensory) could be studied related to specific symptom presentations. Further studies with hypnosis as a model might also shed light on dissociative mechanisms underlying FND.
Several crucial issues were identified and discussed in this review, and recommendations were provided. Multiple diagnostic criteria (e.g., FND, conversion disorder, PNES, PMD) have been used in previous publications. Current DSM-5 diagnostic criteria for FND cut across disciplines, incorporating the range of diagnoses, and should be utilized to allow for valid, comparable, and consistent diagnoses in future research studies.
Conflicting results regarding networks potentially involved in FND were identified in this review, which may be a function of small sample sizes, differences in FND subtypes, and methodological or task differences. Understanding the similarities and differences between subtypes may help resolve these apparent conflicts. Studies can be designed to focus on specific semiologically homogeneous presentations and compared with alternate presentations (e.g., motor FND versus sensory FND) based on specific hypotheses. Alternatively, studies can consider semiologically heterogeneous symptoms as a group to assess the role of commonalities between presentations, given that many patients with one form of a FND have another type (e.g., a significant number of patients with PNES also have PMD). The pathophysiology of acute compared with chronic symptoms and active versus remitted symptoms may also differ.
Many published studies were limited by sample size. To increase sample size, multicenter studies should be considered among specialty centers, as has been done for PNES.
170 The issue of appropriate controls should be considered. The inclusion of organic neurological disorders or other psychiatric disorders (e.g., dissociative disorders or anxiety disorders) as a control could allow the identification of disease-specific characteristics. One intervention is being used for both ES and PNES
171 and can address systemic and somatoform disorders.
Because most of the neurophysiological studies reviewed here are cross-sectional in nature, whether these observations are state or trait related is not clear; studies should begin to address whether a finding is a state- (e.g., secondary to the symptom or lack of use) or trait-related effect (e.g., predictor or causal of FND). The formulation of models and testable hypotheses, recognizing that multiple pathways might converge to a final common presentation, allows the appropriate study design. Understanding similarities and differences in the types of FNDs may lead to other treatments for patients with these disorders.
Acknowledgments
The authors thank Dr. Mark Hallett for helpful comments on the manuscript.