Apathy may be described as a syndrome characterized by decreased interest or initiative, poor persistence in activities, restricted engagement in social interactions, and/or emotional indifference.
1 Such motivational deficits are common in schizophrenia and have been associated with poor compliance with or inability to benefit from treatment,
2 functional outcomes,
3,4 and quality of life.
5A growing neuroimaging literature in neurological populations supports an association between apathy and integrity of frontal-subcortical circuitry.
6–8 In patients with schizophrenia, apathy has been reported to be associated with lower resting cerebral blood flow in the frontal lobe,
9 smaller volume of the frontal lobes bilaterally,
10 as well as abnormalities of the cortical thickness and white matter connections of the left orbitofrontal cortex and left anterior cingulate cortex.
11,12 In addition, apathy has been observed to be related to greater impairment on neuropsychological measures sensitive to frontal-subcortical circuitry dysfunction in both first episode
13 and chronic
10 patients.
Among subcortical regions, the ventral striatum (VS) has been receiving increasing attention as a possible substrate of apathy.
14,15 The VS has been reported in human and animal studies to play a role in emotion and goal-directed, motivated behavior including the processing of reward.
16,17 Structural MRI studies have observed that smaller volume of the VS is associated with greater apathy in HIV-positive adults,
18 patients with frontotemporal dementia,
19 and in those with Parkinson’s disease.
20 A positron emission tomography study of depressed patients with Parkinson’s disease found that greater apathy was associated with lower dopamine and noradrenergic transporter binding in the VS.
21 Furthermore, functional MRI (fMRI) studies have observed that greater apathy, but not other negative symptoms, was associated with reduced activation of the VS during anticipation of monetary reward in patients with schizophrenia.
22–24In the present investigation, we used MRI and voxel-based morphometry (VBM)
25 to examine the hypothesis that apathy is associated with reduced gray matter volume in the VS of patients with schizophrenia. Such a VBM volume-of-interest approach has been implemented in previous research on schizophrenia.
26 A measure of depression was included as a covariate in the analysis given the partial overlap of the symptoms of apathy and depression.
27Methods
Participants
Participants consisted of 23 patients with DSM-IV diagnoses of schizophrenia (N=14) or schizoaffective disorder (N=9), recruited from outpatient community clinics through referral to the study by a treatment provider. The sample had a mean age of 39.43 years (SD=12.28), 52% were men, 87% were right handed, and mean parental education was 13.07 years (SD=3.79). Diagnosis was established using the Structured Clinical Interview for DSM-IV-TR.
28Participants were excluded if they had a history of neurological disorder (e.g., epilepsy, neurodegenerative disorder), mental retardation, or significant systemic medical illness. In addition, patients were excluded if they had a history of substance abuse within the 6 months before participation. All patients were receiving stable doses (at least 12 weeks) of antipsychotic medication at the time of the study (atypical only, N=20; both atypical and typical, N=2; typical only, N=1), with most taking multiple medications. After complete description of the study, written informed consent was obtained following a protocol approved by the Dartmouth College Committee for the Protection of Human Subjects.
Procedure
We assessed severity of clinical symptoms using the Scale for the Assessment of Positive Symptoms (SAPS)
29 and Scale for the Assessment of Negative Symptoms (SANS)
30 and assessed depression using the Beck Depression Inventory–II (BDI-II).
31 The Apathy Evaluation Scale (AES) was used to assess self-reported apathy.
32 The AES is an 18-item questionnaire that is rated on a 4-point scale ranging from “not at all” to “a lot,” with scores ranging from 0 to 54, where higher scores indicate greater apathy.
MRI Data Acquisition
Scans were acquired with a GE Signa 1.5-T Horizon LX magnet (General Electric, Fairfield, Conn.) with echo speed gradients using a standard RF head coil. A T1-weighted three-dimensional spoiled gradient echo (SPGR) coronal volume was acquired. Parameters were TR=25 ms, TE=3 ms, flip angle=40 degrees, NEX=1, and slice thickness=1.5 mm (no gap), yielding 124 contiguous slices with a 24-cm field of view, and a 256×256 matrix with 0.9375 mm in-plane resolution. A fast spin echo T2-weighted scan was also acquired to screen for focal lesions or other incidental findings (TR=3000, TE=96, 3-mm contiguous axial slices).
MRI Data Processing
We performed VBM
25 using the statistical parametric mapping package (SPM12; Wellcome Department of Imaging Neuroscience, London, UK). This involved automated segmentation of gray matter, white matter, and CSF in the SPGR data, nonlinear registration of all images to the Montreal Neurological Institute (MNI152) template space via a data-derived gray matter/white matter–based template of 1.5 mm
3 isotropic resolution created using Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL),
33 with modulation to preserve tissue volumes and spatial smoothing (full width at half maximum=4 mm).
Several steps to ensure accurate segmentation were completed, including visual inspection of raw images, visual inspection of gray matter, white matter, and CSF segmentations, and comparison of the gray matter-to-white matter ratio and total brain volume across participants. One scan was discarded based on poor raw image quality (leaving N=23 for analysis). Smoothed normalized gray matter maps were used for statistical analysis after a final visual inspection of segmentation quality.
To examine gray matter volume in the VS, we performed an analysis using a volume of interest (VOI) specified by a voxel mask from a publication-based probabilistic atlas.
34 The probabilistic VOI mask was thresholded at the 0.5 probability level, which had been previously used in fMRI studies of the VS in schizophrenia,
35 to create a binary mask.
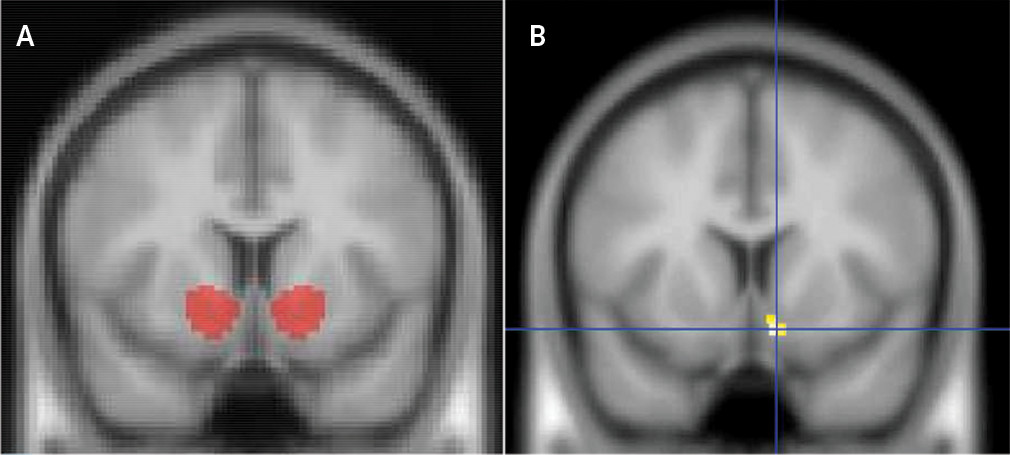
Figure 1 [A] illustrates the anatomic boundary of the VOI on the MNI152 template brain.
Statistical Analysis
We conducted statistical parametric mapping, restricted to the VOI, on a voxel-by-voxel basis using a general linear model and random-effects analysis as implemented in SPM12. Multiple regression analysis was used to examine the correlation between apathy score and gray matter volume in the VOI controlling for age, gender, BDI-II score, and total gray matter volume. A statistical threshold for significance of p<0.01 was implemented without correction for multiple comparisons given the focused VOI approach, although the PWE-corrected p value is also presented for interested readers. Data are presented in MNI152 atlas coordinates.
Results
The sample had a mean AES score of 14.43 (SD=10.11, range=1–48) indicating a relatively mild level of apathy. SAPS (mean=7.0, SD=3.5) and SANS (mean=10.6, SD=3.8) scores were also generally in the mild range. BDI-II scores were typically in the minimal to mild range (mean=13.3, SD=11.6).
VBM indicated that greater apathy was associated with smaller gray matter volume in the right VS VOI [Z=3.67;
Puncorrected=0.001, k=21; peak (xyz) = 9, 10, –14; cluster-level p
FWE of 0.051] (
Figure 1 [B]). Furthermore, an exploratory whole-brain analysis also produced a significant association of apathy and right VS volume (Z=3.60, p=0.001, k=174), albeit only when uncorrected for multiple comparisons. No significant correlation was observed between apathy and left VS volume. No significant association was observed between VS volume and either BDI-II score or chlorpromazine-equivalent medication dosage.
Discussion
The present investigation revealed an association between greater self-reported apathy and reduced gray matter volume in the right VS, particularly the nucleus accumbens, in patients with schizophrenia. This relationship was observed despite statistically controlling for depression, consistent with other work showing that apathy can be dissociated from depression.
36–38 Our finding is also in line with investigations in other patient populations that have implicated abnormality of the VS in apathy.
18,20,21 Furthermore, we observed that apathy was related specifically to right VS volume. Such an association between apathy and the right VS has previously been observed in studies of schizophrenia
22 and frontotemporal dementia.
19 The precise functional significance of this lateralization, however, remains to be elucidated.
A number of authors have proposed that the pathophysiology of apathy involves the VS given the region’s involvement in motivation and emotion.
14,15 fMRI studies have reported that patients with schizophrenia show reduced responsiveness to behaviorally salient stimuli in brain regions involved in motivation, such as decreased activation of the VS and related basal forebrain regions in response to target stimuli during an auditory oddball task,
39 and reduced activation of the VS, including the right VS, to cues signaling the possibility of obtaining a monetary reward.
22–24,40 These findings along with that of the present study suggest that apathy in schizophrenia may be due in part to deficient processing of stimuli signaling the potential for reward, or a more general dysfunction in processing motivationally significant stimuli, resulting from structural and functional abnormalities of the VS and related circuitry. Involvement of the VS is also consistent with the affective/motivational subtype of apathy proposed by Levy.
14 In that model, it is argued that abnormality of basal ganglia subregions such as the VS and the orbital and mesial prefrontal cortices leads to difficulty associating affective/emotional signals with ongoing and future behaviors, consequently resulting in diminishing motivation.
It is important to note that it is unlikely that VS abnormality fully accounts for apathy in schizophrenia. Case studies involving lesions restricted to the VS are rare and have not been associated with the presence of apathy.
41 In a similar manner, behavior akin to apathy has not been consistently reported following focal lesions to the VS in primates.
42–44 Previous research has implicated the frontal lobes in apathy in schizophrenia
10–12 and in neurological disorders.
19,45 It is therefore possible that frontal abnormality is playing a role in driving dysregulation of the VS in schizophrenia. A recent fMRI study that observed normal VS response, but abnormal striatal-cortical connectivity to motivational cues that was related to negative symptom severity in patients with schizophrenia, may support that hypothesis.
46 A more complete understanding of the etiology of apathy in schizophrenia will therefore require further investigation of the structural and functional integrity of subregions within the frontal-striatal circuitry, as well as functional connectivity of the circuitry.
The results of the present investigation must be interpreted within the context of its limitations. The sample size was modest; therefore, the findings require replication in a larger sample, which should also evaluate whether they are upheld when considering subtypes of the disorder, gender, and handedness. The relationship observed between apathy and VS volume was significant using a focused volume-of-interest approach and is consistent with previous studies, but it was nonetheless a relatively small effect. This may be due in part to the overall mild severity of apathy in the sample. It is possible that we would observe an even stronger relationship in patients with schizophrenia having more severe levels of apathy. Further study is also needed to determine whether antipsychotic medication could have affected the findings, although the latter seems unlikely given that we found no association between VS volume and medication dosage, and a previous study found a relationship between apathy and functional activation of the VS in patients with schizophrenia taking atypical antipsychotics.
22 Finally, although we did not have information pertaining to duration of illness in our patient sample, it is unlikely that this variable can account for our results given that other work has found that duration of illness is unrelated to volume of the accumbens in patients with schizophrenia.
47With those caveats, the present findings provide further support for the hypothesis that apathy in schizophrenia is associated with abnormality of neural circuitry subserving motivated behavior. Such findings could inform the treatment of apathy, as well as raising the possibility of using a neuroimaging marker (i.e., abnormal structure and/or function of the VS) to identify patients who may be at elevated risk of experiencing apathy and thus provide early intervention.