Application of functional MRI (fMRI) in the ictal period is constrained by practical limitations: it is impossible to predict when seizures will occur except in cases of special epileptic syndromes that can be deliberately induced, such as musicogenic epilepsy.
6 As an alternative, resting-state fMRI (rsfMRI) has been used simultaneously with electroencephalography (EEG) in the interictal period
7 to infer causality related to seizure initiation and propagation.
8 However, such a combined approach is not generally available or technically easily feasible, thus making it worthwhile to assess whether rsfMRI alone can be used to investigate clinically relevant aspects of epilepsy.
9Graph-theoretical analysis has been used successfully to identify abnormalities of network configuration in patients with various neuropsychiatric diseases.
13–15 In TLE, changes in brain functional networks in the interictal state have been characterized as disruption to functional integration over the whole brain.
16–18 In the present study, we investigated the distribution of interictal abnormalities in TLE patients in terms of alterations in functional integration at the regional level, as well as hemispheric and whole-brain levels, to elucidate the clinical relevance of disrupted brain functional integration. We hypothesized that the extent of disrupted brain functional integration could be related to seizure control status and/or comorbid cognitive dysfunction, specifically memory impairment.
Methods
Subjects
The study subjects were 48 patients with TLE (mean age: 41.4 years [±12.6 years], 31 females) and 45 age- and gender-matched healthy controls (mean age: 41.2 years [±13.5 years], 23 females). Epilepsy was diagnosed clinically and based on detailed laboratory investigations, including long-term video EEG monitoring, structural and functional brain imaging, and neuropsychological evaluations. The clinical diagnosis of TLE was made according to criteria of the International League Against Epilepsy.
19 Lateralization of the seizure focus was determined in the traditional manner by assessing seizure semiology, epileptiform discharges on ictal or interictal EEG, and hippocampal sclerosis or atrophy on structural MRI. Mean age at epilepsy onset was 24.6 years (±13.0 years), and the duration of epilepsy was 17.0 years (±12.7 years). Among the 48 TLE patients, 26 patients were determined to have left TLE (LTLE), and the other 22 patients had right TLE (RTLE). All healthy controls had no history of neurological disorders and no abnormalities on structural MRI. Written, informed consent was obtained from all participants in accordance with the Declaration of Helsinki and its later amendments, and the study protocol was approved by the Ethical Committee of Ewha Medical Center, Seoul, Korea.
Assessment
Seizure control status was assessed based on the Engel classification.
20 Each patient was assigned to one of four classes: class I, seizure free or auras only; class II, no more than twice per year; class III, no more than once per month; and class IV, more than once per month. Based on this classification, we divided the TLE patients into two subgroups: 1) a good seizure control (TLE
GSC) group consisting of the class I and II patients and 2) a poor seizure control (TLE
PSC) group consisting of the class III and IV patients.
Neuropsychological tests were performed to assess verbal memory with a Korean version of the California Verbal Learning Test (K-CVLT)
21 and nonverbal memory with the Rey-Osterrieth Complex Figure Test (ROCFT).
22 For each test, immediate recall (IR), delayed recall (DR), and recognition (R) scores were recorded and adjusted for age and education level.
Acquisition of MRI Data
MRI data were acquired with a 3 Tesla Achieva system (Philips, Best, the Netherlands). For each subject, 150 volumes of rsfMRI data were obtained with blood-oxygen-level-dependent (BOLD) contrast in the axial plane using a T2*-weighted gradient echo echo-planar imaging sequence: repetition time=2,000 ms, echo time=32 ms, voxel size=1.67 mm×1.67 mm×5.00 mm, matrix size=144×144, and slice number=32. During scanning, subjects were instructed to close their eyes and remain as motionless as possible. Structural MRI data were obtained in the coronal plane using a three-dimensional sensitivity encoding sequence: voxel size=0.50 mm×0.50 mm×1.00 mm, matrix size=448×448, and slice number=160.
Preprocessing of rsfMRI Data
Preprocessing of rsfMRI data were performed using the routines in SPM8 (
http://www.fil.ion.ucl.ac.uk/spm/) and DPARSF (
http://rfmri.org/DPARSF/). The preprocessing steps were performed in the order of spatial realignment for correcting for head movement, normalization into a coordinate frame matching that of a template brain conforming to the Montreal Neurological Institute space, and spatial smoothing with a Gaussian kernel of 4-mm full width at half maximum. Transformation parameters for spatial normalization were derived from segmentation of the high-resolution structural image coregistered to the mean functional image.
In addition, linear trends mainly due to systematic increases or decreases in signal were removed, and nuisance covariates including six head movement parameters estimated during spatial realignment and cerebrospinal fluid and white matter signals were regressed out to reduce the effects of head motion and nonneuronal fluctuations. The functional images were then band-pass filtered at 0.01 Hz–0.08 Hz to reduce the effects of very low- and high-frequency physiological noise.
Construction of Brain Functional Networks
A brain functional network was constructed by defining nodes and estimating edges between them. Nodes were defined in 90 brain parcellations of the whole-brain gray matter determined by the automated anatomical labeling brain atlas.
23 The list of the 45 brain parcellations in each hemisphere is presented in Table S1 in the
data supplement accompanying the online version of this article. Following the procedure in SPM8, the principal or first eigenvariate from singular value decomposition of the voxel signals within each brain parcellation was assigned as the representative signal of each node. Edges were estimated by calculating the Pearson’s correlation-based functional connectivity between the representative signals of the 90 nodes.
Functional connectivity between all pairs of the 90 nodes composed a 90×90 matrix of correlation coefficients, which was then converted to adjacency matrices with a range of sparsity by applying a set of threshold values. Each adjacency matrix represented a brain functional network with unweighted and undirected edges in such a way that 1s corresponded to presence of edges, and 0s corresponded to absence of edges. To increase the number of patients in the subgroups defined by seizure control status, RTLE and LTLE patients were grouped together by flipping the functional brain networks of RTLE patients at the midsagittal plane such that the affected hemisphere (AH) was placed on the left for all TLE patients.
Calculation of Average Efficiency
We wanted to reveal abnormalities in terms of disrupted functional integration in the interictal state of TLE patients. We employed the network parameter “efficiency” (for details, see the Methods section in the online data supplement) to assess the functional integration of brain networks.
The efficiency can be calculated for each pair of nodes in a functional brain network. For any subnetwork composed of nodes, the average efficiency is calculated as the average of efficiency for every pair of nodes in the subnetwork.
We calculated the average efficiency at hierarchical spatial levels (see Figure S1 and Table S2 in the online data supplement): at the whole-brain level, the hemispheric level, and the regional level. In particular, we were interested in searching for the extent of altered average efficiency at the regional level. We therefore specified 296 subnetworks according to 12 brain regions in each hemisphere: central (Cnt), lateral frontal (FrnL), medial frontal (FrnM), orbital frontal (FrnO), temporal (Tmp), lateral parietal (PrtL), medial parietal (PrtM), lateral occipital (OccL), medial occipital (OccM), limbic (Lmb), insular (Ins), and subcortical (Sbc) regions.
Comparison of Average Efficiency
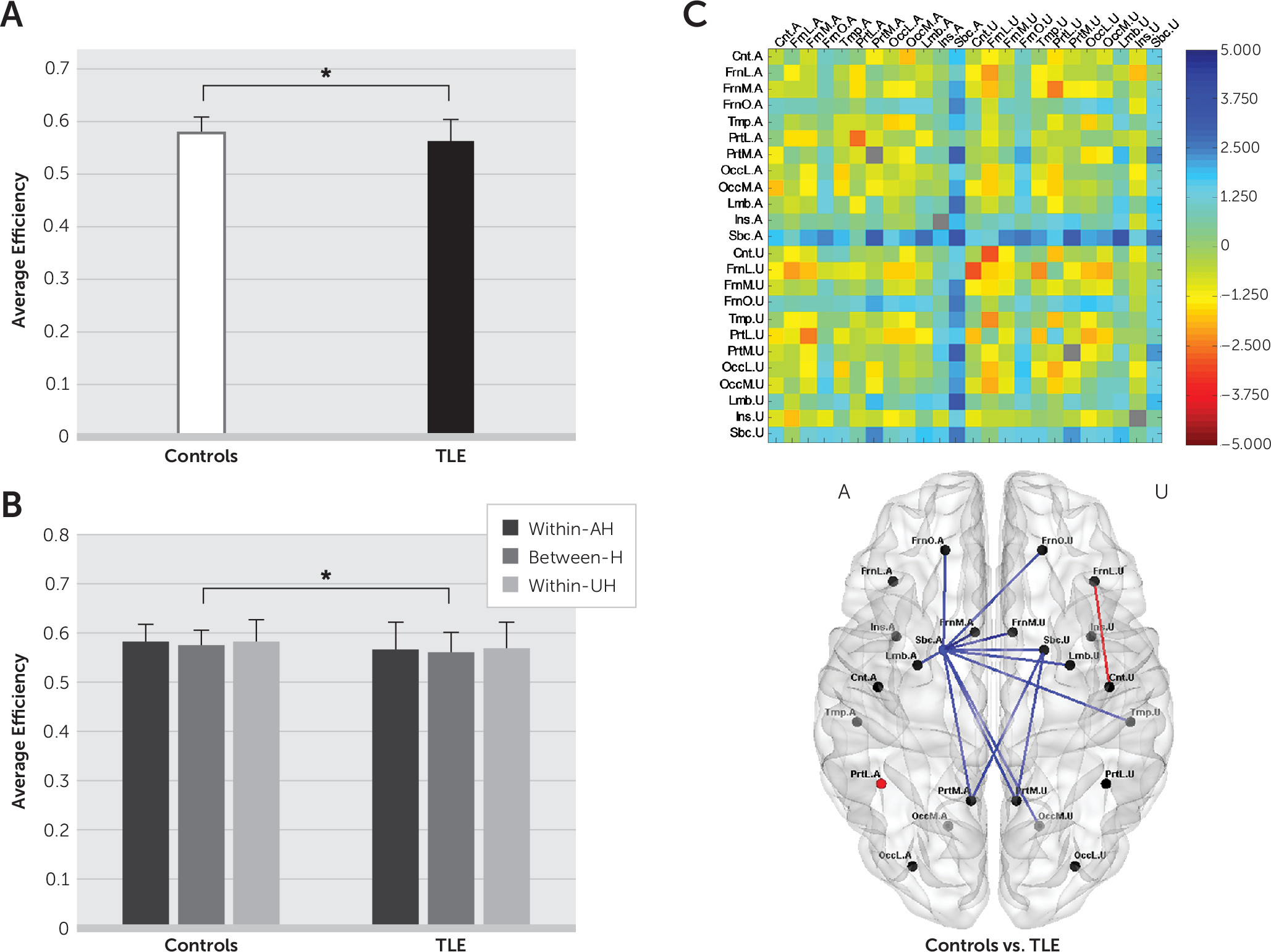
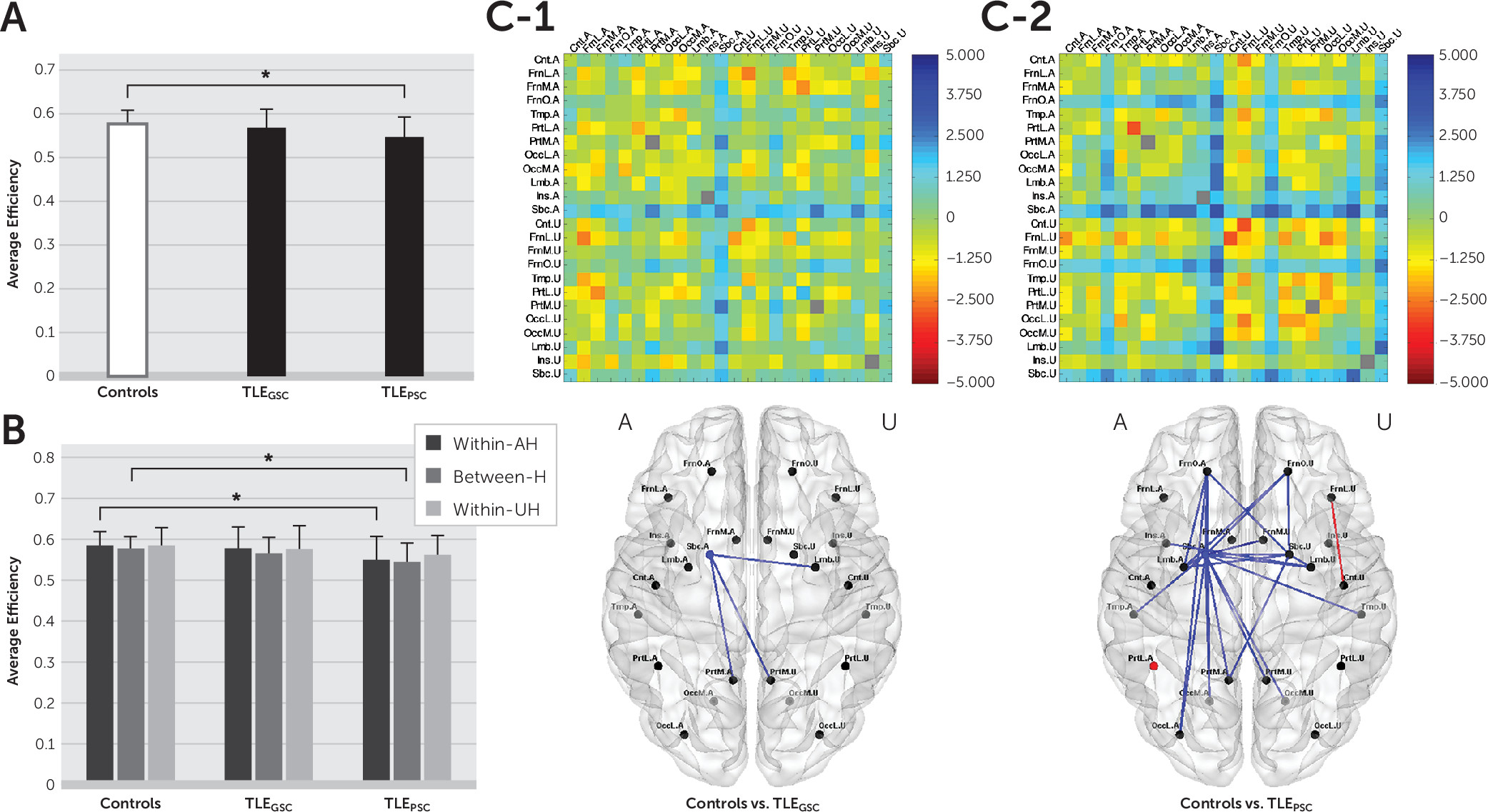
Abnormalities of brain functional networks in epilepsy were revealed by comparing average efficiency between healthy controls and TLE patients at each spatial level. Cost-integrated measures of average efficiency (see the Methods section in the online data supplement) were compared using two-sample t tests for each subnetwork. At the whole-brain level, we checked whether the average efficiency was altered in TLE patients compared with healthy controls and whether such changes were dependent on seizure control status. That is, we conducted a comparison between healthy controls and all TLE patients and then compared the healthy control group with the TLEGSC and TLEPSC subgroups. At the hemispheric and regional levels, we examined which subnetworks contributed to the whole-brain changes.
Furthermore, to examine how seizure lateralization might influence topological changes according to seizure control status, we checked whether seizure lateralization (LTLE and RTLE) interacted with seizure control status (GSC and PSC) by conducting two-way analysis of variance. In the inferences, statistical significance was determined with the p value set at 0.05, corrected for multiple comparisons at each spatial level by following the rough false discovery rate procedure.
Relationship Between Average Efficiency and Memory Performance
The association between average efficiency values and scores on individual measures of the K-CVLT and ROCFT was assessed using Spearman’s rank correlation at each spatial level. We were interested in whether the extent of altered average efficiency involved memory impairment. Thus, we sought correlations within the mask of group differences in the average efficiency, only for subnetworks in which the average efficiency was altered in TLE patients compared with healthy controls. Statistical significance was determined with the p value set at 0.05, corrected for multiple comparisons at each spatial level.
Discussion
In this study, we used rsfMRI to examine the distribution of interictal abnormalities in TLE patients, as measured by alterations in functional integration at the regional level, as well as the hemispheric and whole-brain levels. We demonstrated that 1) brain functional integration was disrupted in TLE patients, more extensively at the regional level in TLEPSC patients than in TLEGSC patients, and 2) there was an association between greater reduction in brain functional integration and more severe memory impairment in regional subnetworks with abnormalities in TLEPSC patients.
Graph theoretical analysis allowed the characterization of topological changes in brain functional networks in TLE and provided evidence that TLE is a disorder of abnormal brain networks, rather than a focal epileptogenic abnormality. Our finding of reduced average efficiency at the whole-brain level is in agreement with previous observations from graph theoretical analysis of fMRI or electrophysiological data in TLE,
16–18 which included observations of increased average shortest path length or decreased average efficiency over the whole brain. Such disruption to brain functional integration may be related to a shift in connection topology to a regularized pattern
16,17 or to an accompanying disruption to functional segregation
18 in TLE.
We further demonstrated that topological changes in functional brain networks were dependent on clinical severity as reflected by seizure control status. In TLEPSC patients, but not in TLEGSC patients, a reduction in the average efficiency was exhibited at the whole-brain level, which could be attributed to declines in the average efficiency within the AH and between hemispheres at the hemispheric level. For TLE patients included in this study, the ratio of GSC to PSC was not statistically different between LTLE and RTLE patients. Thus, the topological changes in functional brain networks seemed independent of seizure lateralization (see Figure S2 in the online data supplement).
By tracking down sub-brain contributions at the regional level, we revealed the distribution of abnormalities in terms of disrupted functional integration within and between regions. All TLE patients had disruption of functional integration near the AH Sbc, but regional involvement was diffuse in TLE
PSC patients, reaching the frontal, temporal, occipital, and insular cortices, whereas disrupted functional integration was restricted to the limbic and parietal cortices in TLE
GSC patients. Between the two subgroups with GSC and PSC, the difference in the extent of interictal abnormalities at the regional level seems to have determined whether topological alterations were seen or not at the hemispheric level and further at the whole-brain level. TLE is often related to reorganization of extratemporal regions, as well as mesial temporal structures adjacent to the epileptogenic zone.
24 More frequent seizures in TLE patients with poorer seizure control may be caused by, or result in, pathological reorganization of brain connectivity involving a wide extent of abnormalities beyond the mesial temporal structures.
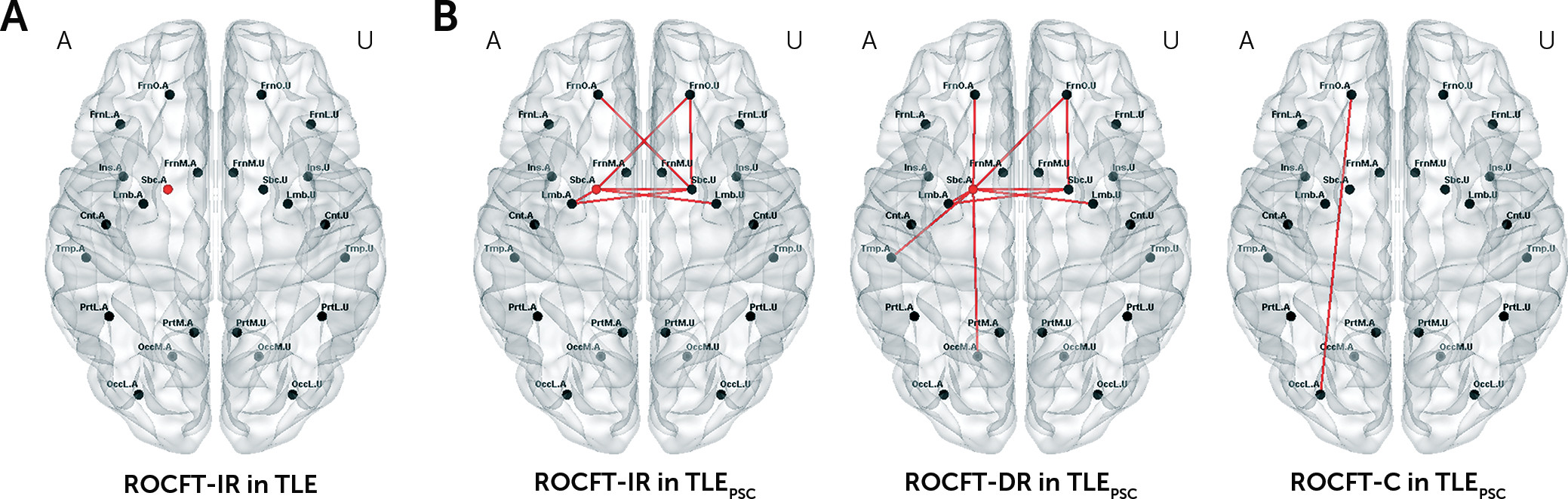
25Together with the wider extent of abnormalities, TLEPSC patients experienced more severe impairment especially in nonverbal memory, as revealed by their ROCFT-IR and ROCFT-DR scores. Furthermore, an association between greater disruption to functional integration and more severe memory impairment was observed only in TLEPSC patients. Brain regional correlates of memory impairment were not completely matched with the distribution of abnormalities, but the wider extent of abnormalities appears to be related to greater vulnerability to memory impairment. Cognitive impairment (e.g., memory impairment) may be concomitant with the extensive distribution of abnormalities as a manifestation of pathological reorganization.
The extent of abnormalities in terms of disrupted functional integration at the regional level seems to be strongly related to clinical severity, including poor seizure control and subsequent severe memory impairment in TLE, although mechanisms underpinning this connection need to be further investigated. We admit that the approaches in this study may not be used directly to make a diagnosis of seizure control status and memory impairment in TLE. Rather than proposing measuring disrupted brain functional integration to predict clinical factors, we hoped to ascertain its applicability and eligibility for being informative about clinical severity. Future steps to incorporate potentially crucial interictal features into a formal prediction model for seizure control or cognitive impairment would be warranted.
When we searched for disrupted brain functional integration in the interictal state, the distribution of abnormalities was mainly observed near the AH Sbc for both TLE
GSC and TLE
PSC patients. Concerning the reason why the AH Sbc was commonly involved in TLE patients regardless of seizure control status, the roles of the amygdala and thalamus in the AH Sbc should be mentioned. Both the amygdala and thalamus are involved in the initiation of seizures in TLE, as mesial temporal sclerosis often involves the amygdala
26 and the thalamus is a critical component of the initiating circuit that supports the beginning of seizures.
27 In addition, alterations in connectivity between the thalamus and other structures have been seen even in the interictal state.
28 Identifying circuits or networks related to seizure initiation and propagation is one of the main concerns in epilepsy.
29 Although interictal abnormalities tend to provide limited information, the sharing of common features of abnormalities between ictal and interictal states
3 still suggests the possibility of applying information about interictal abnormalities to infer the seizure focus and paths of seizure spread.
Several limitations may be raised about the current study. First, it should be noted that the connection topology of brain functional networks is determined based on brain parcellation schemes.
30 Our composition of subnetworks at the regional level may be arbitrary, but it is likely that a range of ways to construct functional brain networks and adequate numbers of subnetworks would produce similar abnormality profiles. Second, we dealt with brain functional networks of all TLE patients by placing the AH in the same side, as we were mainly interested in the effects of seizure control status, conceding a certain vagueness in determining seizure lateralization. In spite of confounding effects related to hemispheric lateralization of brain functions, neuroimaging studies for neurological diseases, such as stroke with unilateral lesions,
31 have often been conducted by locating the AH in the same side. Third, factors that may influence BOLD signals have not been fully taken into account. For example, seizure-related alterations in vascular structures and blood flows could affect BOLD signals, and thus resting-state functional connectivity and functional brain networks. In addition, we used the efficiency as a network parameter of interest on the grounds of consistent previous findings of disruption to functional integration over the whole brain, but alternate network parameters, such as degree
32 and centrality,
33 may be further tested to examine clinical relevance of abnormal connectivity in TLE. Finally, several patients had structural brain lesions, such as hippocampal sclerosis, which might have influenced our results on functional connectivity analysis. We could not thoroughly assess effects of structural alterations on seizure control status or changes in functional brain networks. Such effects should be investigated in a future study including more patients with various structural lesions.
In summary, we investigated clinical relevance of interictal abnormalities based on disrupted brain functional integration in TLE. We demonstrated that more extensive alterations in functional integration within and between brain regions could be related to poorer seizure control and more severe memory impairment. Such clinical relevance of disrupted brain functional integration represents a step toward employing rsfMRI for assessing clinical characteristics, possibly related to treatment outcomes or cognitive prognosis, in TLE patients.