Behavioral variant frontotemporal dementia (bvFTD) is a neurodegenerative disorder that results in behavioral disinhibition, apathy, lack of empathy, compulsive behaviors, changes in eating behavior, and impaired executive functions.
1 Patients with bvFTD manifest emotional blunting, decreased empathic responses to others, and socioemotional behavioral changes.
2 The source of their socioemotional changes is neuropathology in critical frontotemporal areas. These areas control attentional and autonomic systems, and one relatively unexplored association of socioemotional changes in bvFTD is a decrease in the ability to redirect and focus attention on social or emotional stimuli.
The examination of heart rate (HR) responses to arousing pictures among bvFTD patients may clarify whether they have early disruption of the ability to orient attention to social and emotional stimuli.
3 Originally named by Ivan Pavlov as an initial HR orienting response (OR), or “what is it?” reflex, the OR involves the refocusing of attention on novel or significant stimuli.
4 This initial HR deceleration or “attentional bradycardia” facilitates perceptual processing of these stimuli prior to subsequent preparation of the organism for action.
5–8 The OR is primarily mediated by the parasympathetic (vagus) nervous system and is steepest during the first 1–2 seconds of picture presentation.
6,9 ORs are expected when viewing all novel pictures, although the elicitation and magnitude of the OR varies with the potential significance or impact of the stimulus, particularly if emotionally significant but also if socially significant.
6The present study evaluated ORs to social and emotional stimuli among bvFTD patients compared with normal controls (NCs). For stimuli, this study used the International Affective Pictures System (IAPS),
10 a widely used set of emotional picture stimuli with normative data for valence (ranging from pleasant to unpleasant) and arousal (ranging from calm to excited).
10 Among bvFTD patients, this is the first study, to our knowledge, to directly compare social versus nonsocial stimuli while accounting for the affective content (pleasant versus unpleasant) of the stimuli, thus allowing discrimination of the OR effects of sociality separate from the effects of affective valence. Given the neuropathology of bvFTD, we hypothesized that bvFTD patients would exhibit decreased OR responses to the more impactful social and unpleasant stimuli, compared with the nonsocial and pleasant ones.
Discussion
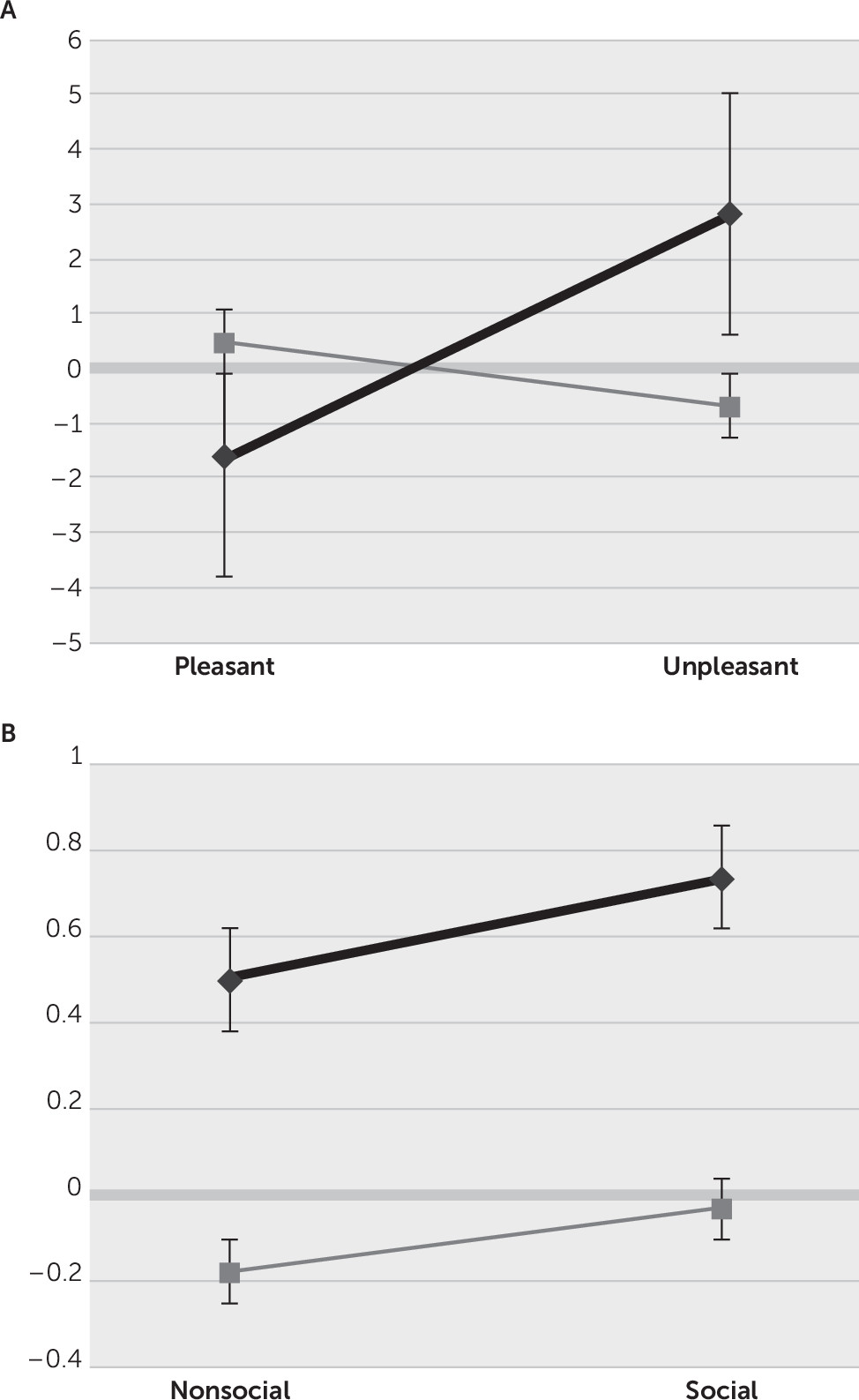
This study investigated attentional orienting responses to socioemotional stimuli among patients with bvFTD compared with NCs. We examined the Pavlovian OR of initial HR deceleration as a measure of perceived importance and registration of social and emotional stimuli. The analysis reveals that NCs, but not bvFTD patients, showed the expected OR to unpleasant pictures, the most arousing of the four stimulus groups. A lack of HR deceleration among the bvFTD patients correlates with the degree of emotional blunting. In these patients, the finding of decreased “attentional bradycardia” may reflect diminished appreciation of the emotional significance of unpleasant stimuli, or, alternatively, the dampened emotions in bvFTD contribute to diminished autonomic attentional reorientation to emotional stimuli.
8,17,18The early location of neuropathology in bvFTD supports the view that socioemotional dysfunction in this dementia may have roots in the inability to appreciate, attend, and respond to stimuli with emotional content.
19 The neuropathology in bvFTD focuses on the major frontal control areas that control attentional changes, such as the anterior cingulate cortex and resting autonomic nervous system (ANS) levels.
2,17,18,20 Other mesial or paralimbic frontal lobe structures affected in bvFTD are involved in controlling or regulating sympathetic and parasympathetic responses, including the ventral mesial prefrontal cortex and its connection to the amygdala for vagally mediated HR deceleration.
21,22 Moreover, patients with bvFTD have decreased ANS responses to unpleasant emotions, including disgust and fear conditioning to aversive stimuli,
23,24 which also seems to correspond to emotional blunting.
The OR may be an excellent reflex for assessing perceived alterations in the socioemotional significance of stimuli. The OR is an ANS-mediated reflex relating attention and focused perception on stimuli with emotion and interoceptive awareness, particularly if negative or threatening.
25 In previous studies, healthy adults subjected to emotionally unpleasant stimuli have shown greater initial ORs, when compared with neutral or pleasant stimuli.
6,16,26–28 In contrast, the nonthreatening emotional aspects of pleasurable stimuli may not require as much of an initial attentional reorientation; hence, they may have minimal ORs and faster onset of sympathetic reactivity with early increased HRs, as in our NCs.
3,6,29–31 Paradoxically, although not statistically significant, the bvFTD patients showed an OR to pleasant stimuli, possibly reflecting their increased drive to orient to appetitive items.
1 This HR acceleration, often generally termed a “defense reaction” but also an appetitive reaction, follows the OR when present and actually reflects sympathetic ANS reactivity in preparation for action.
26,27,32 In bvFTD, the mitigation of ORs and the tendency to go rapidly to sympathetic reactivity after viewing unpleasant stimuli is consistent with the neuropathology and symptoms of bvFTD and may be a good measure for distinguishing these patients early in their course.
33This study showed that, compared with NCs, patients with bvFTD had impaired ORs to the unpleasant emotional content of the stimuli but not to their social content. Although there have been few prior studies on ORs to social (images of people in various situations) stimuli, differential orienting to social versus nonsocial stimuli is relevant to survival, reproduction, and social life,
34 and prior studies have shown varying responses to social stimuli depending on their “sociality”
34,35. Differential responses to social stimuli, however, could actually be consequent to emotional arousal and not to social content, as in the bvFTD patients in this study.
6,26,36–38 For example, in one study, exposure to social positive film clips resulted in larger skin conduction responses (SCRs), a sympathetic rather than OR measure, compared with nonsocial positive clips, but nonsocial negative clips resulted in larger SCRs than social negative clips,
38 and in another study, SCRs increased on presenting negative social images (e.g., mutilation), compared with positive social images.
39 The present study did not find a significant effect of social versus nonsocial pictures, perhaps because of the lesser importance of reorienting attention to social stimuli compared with emotional valence.
In bvFTD patients, emotional blunting appears to correlate with their decreased initial orienting but does not establish directionality. Further studies with larger samples can establish cause and effect (i.e., whether critical frontal pathology in bvFTD results in dampened responses to the emotional aspects of stimuli with decreased ORs or whether decreased ORs contribute to the dampening of emotions). The presence of an OR response to pleasant stimuli among these patients suggests that the lack of autonomic response to emotional stimuli could drive the affective blunting. Establishing this directionality could have major implications for prevailing theories of emotion.
Although the present study shows OR differences in bvFTD compared with NCs, there are a number of potential limitations. First, the sample sizes are relatively small. Despite this, our investigation does show several significant alterations in OR among the bvFTD patients compared with NCs. Second, a potential limitation is the lack of participant ratings of the IAPS stimuli for valence and arousal. The brief and sequential presentations did not permit subjective ratings of how the stimuli made the participants feel. Moreover, studies involving bvFTD patients document the unreliability and correspondence to stimuli of their verbal or subject reports and ratings.
33,40 Finally, this preliminary study could not exclude more general effects of having dementia, and subsequent work needs to compare bvFTD with other dementia comparison groups.
In conclusion, this preliminary investigation discloses alterations in the initial orienting to socioemotional stimuli in bvFTD patients. These patients have decreased and variable attentional orienting to these stimuli, primarily to the emotional, unpleasant aspects of pictures, prior to initiating a sympathetic response. In addition to clarifying the relationship of ORs to emotion, future studies can explore whether impaired ORs could be an early diagnostic marker of bvFTD.