Trichotillomania, also known as hair pulling disorder, is a body-focused repetitive behavior characterized by compulsive pulling of hair, leading to hair loss and marked functional impairment.
1 Trichotillomania is associated with a significant degree of psychosocial dysfunction, poor quality of life, and medical complications and appears to be a fairly common disorder in the general population. Small prevalence studies have found point prevalence rates ranging from 0.5% to 2.0%.
2 From a phenomenological perspective, trichotillomania is characterized by repetitive and excessive maladaptive grooming habits that are difficult for individuals to suppress.
3With only three functional neuroimaging studies published in trichotillomania, there is scant information regarding the neurobiological mechanisms underlying this disorder. One study failed to find any significant differences in implicit learning or in striatal or hippocampal activation using a serial reaction time task in 10 adults with trichotillomania compared with 10 healthy controls.
4 Another study of 21 adults with trichotillomania found dampening of nucleus accumbens responses to reward anticipation (but relative hypersensitivity to gain and loss outcomes) compared with 14 healthy controls.
5 Finally, a study of nine children with trichotillomania, compared with 10 healthy controls, found that those with trichotillomania exhibited significantly greater activation in the left temporal cortex, dorsal posterior cingulate gyrus, and putamen during a visual symptom provocation, and greater activation in the precuneus and dorsal posterior cingulate gyrus during a visual and tactile provocation.
6Trichotillomania has long been considered an obsessive-compulsive spectrum disorder, given the overlapping phenomenological and clinical characteristics of trichotillomania and obsessive-compulsive disorder (OCD). Patients with OCD often show behavioral impairments in inhibitory control and flexible responding;
7 therefore, the orbitofrontal cortex (OFC) is central to our understanding of OCD as it subserves reversal learning, a cognitive function wherein behavior is flexibly altered after negative feedback.
8 Adults with OCD have shown abnormally reduced activation of the lateral orbitofrontal cortex during reversal learning. These deficits extend to their first-degree relatives who are clinically asymptomatic, highlighting the centrality of this cognitive function and its implicated neural substrates in the pathophysiology of OCD.
9 Additionally, in a small study of pediatric trichotillomania (N=16), youths with hair pulling disorder demonstrated significant deficits in several areas of executive functioning performance, including reversal learning.
10 Difficulties in behavior reversal may help explain pulling persistence despite negative consequences of pulling (e.g., social isolation, poor self-esteem). Furthermore, one study in skin picking disorder (a disorder with shared pathophysiology with trichotillomania) found that impaired cognitive flexibility was a potential marker of response to treatment with a glutamate modulating agent.
11 As such, functional MRI (fMRI) tasks examining cognitive flexibility may be a useful means of probing frontal orbital and dorsolateral functioning in disorders related to OCD, such as trichotillomania. The objective of this study was to probe dorsolateral orbitofrontal cortex functioning in people with trichotillomania compared with matched healthy controls using an fMRI cognitive flexibility paradigm. In line with previous research,
9 we hypothesized that trichotillomania would demonstrate dysfunction during reversal learning, as evidenced by hypoactivation of the orbitofrontal cortices.
Methods
Study Participants
Men and women aged 18–54 years with a primary diagnosis of trichotillomania based on DSM-5 criteria and a structured clinical interview with a board-certified psychiatrist with expertise in trichotillomania and body-focused repetitive behaviors were recruited by newspaper and poster advertisements. All participants were recruited and underwent neuroimaging procedures at the University of Chicago.
Individuals were included in the trichotillomania group if they had a current primary diagnosis of trichotillomania and no contraindication to MRI. Exclusion criteria were unstable medical illness; current pregnancy or use of inadequate contraception; thoughts of suicide; history of bipolar disorder, dementia, or psychotic disorder; substance use disorder in the past 12 months; initiation of behavior therapy or psychotropic medications within the past 6 months; and current use of illicit drugs based on urine toxicology.
Healthy control subjects were recruited via media advertisements on the basis of no history of psychiatric disorders and no known history of trichotillomania or obsessive compulsive related disorders (e.g., OCD, excoriation disorder, body dysmorphic disorder) in first-degree family members.
The study procedures were carried out in accordance with the ethical standards laid out in the latest version of the Declaration of Helsinki. The institutional review board of the University of Chicago approved the study and the consent statement. After complete description of the study to the subjects, written informed consent was obtained.
Assessments
All study subjects first received a psychiatric, medical, and family history evaluation. Clinical instruments included the Mini-International Neuropsychiatric Inventory (MINI),
12 the Massachusetts General Hospital Hairpulling Scale,
13,14 and the Quality of Life Inventory.
15Imaging
After completion of the above, participants undertook high-resolution structural imaging using a 3-T scanner. A total of 310 T2*-weighted volumes depicting blood oxygen level-dependent (BOLD) signal were acquired; the first 10 were discarded to avoid T1-equilibrium effects, using the following parameters: TR=2 seconds; TE=30 ms; 32 axial 3 mm; three slices; field of view=192×192 mm; 64×46 matrix.
The intradimensional extradimensional (IED) fMRI task has previously been validated in healthy volunteers
16 and in adults with OCD.
9 The reader is referred to these previous studies for a complete description of the task. In brief, the paradigm was derived from the principles of the Wisconsin Card Sorting Test and was designed to decompose different aspects of learning and flexible responding. On each trial, participants view two stimuli, each made up of a superimposed image of a face and a house. Through trial and error, the participant attempts to work out an underlying rule about which stimulus is correct. Positive feedback (the word “correct”) is presented in approximately 75% of trials in which the subject responded correctly; no negative feedback is given. Trials such as these provide an ideal cognitive baseline relative to other trials where the subject is searching. After the volunteer has made several correct responses, the underlying rule about the correct stimulus is changed, or novel stimuli are introduced. For the present study, the two key components of the task were reversal of responses and extradimensional set-shifting. On reversal trials, the relevant stimulus dimension remains the same, but the correct response changes to the alternative one; for example, if the relevant dimension was the appearance of the house, then the rule changed so that the previously correct house became incorrect, and participants had to learn to select the other house. On extradimensional set-shifting trials, two new stimuli are introduced, and the relevant dimension changes; for example, if the appearance of the house had been the basis for making a correct decision, two new stimuli were presented, and the relevant stimulus dimension for making the correct choice became the faces rather than the houses. Across species, reversal learning is dependent on the orbitofrontal cortices, whereas extradimensional shifting is dependent on the lateral prefrontal cortices.
8,16 Other aspects of the task included intradimensional set-shifting trials, in which new stimuli were shown but the relevant dimension for getting the rule correct remained unchanged; for example, if the appearance of the houses was important, then this remained the case following an intradimensional shift.
fMRI recordings were preprocessed and analyzed using SPM12. In brief, whole brain volumes were motion-corrected, slice-time acquisition corrected, coregistered to the structural scan, normalized to Montreal Neurological Institute (MNI) space, and smoothed with an 8 mm full-width at half-maximum Gaussian kernel. The intradimensional, extradimensional switches, reversals, stimuli set changes, and simple correct (no switching) events were convolved with the canonical hemodynamic response function and modeled alongside the 24-parameter motion estimates
17 as nuisance regressors within the subject level general linear models to compute the contribution of each condition to the BOLD signal. Subject-level contrast estimates for each condition (switch events) were then calculated relative to the cognitive baseline correct trials (no switching) and elevated to the group level.
Group-Level Analysis
The subsequent analyses focused only on reversal and extradimensional switch events, as these were our primary measures of interest. For both events, independent random effects analyses were conducted with one-sample t tests using the contrast estimates from all subjects across both groups. The resulting group-level activation maps for each contrast were thresholded, voxel-wise, at a p value of 0.001, uncorrected, and subsequently cluster corrected for multiple comparisons using a p value <0.05, false discovery rate. The approach applied for localizing regions active during certain tasks in the present study is well practiced and considered standard practice.
18Cluster-corrected group activation maps were segmented into independent regions of interest using a custom three-dimensional watershed algorithm. Briefly, this approach segments continuous statistical spaces into discrete parcels via an expanding voxel neighborhood search that initializes at the local minima of an inverted statistical map and iterates until voxels from independent local minima are met. Mean beta estimates were extracted for each region of interest across subjects and examined at the group level. As this was an exploratory study in a relatively small number of participants, group-level differences in regional activations were assessed with uncorrected two-tailed independent samples t tests (α=0.05).
Discussion
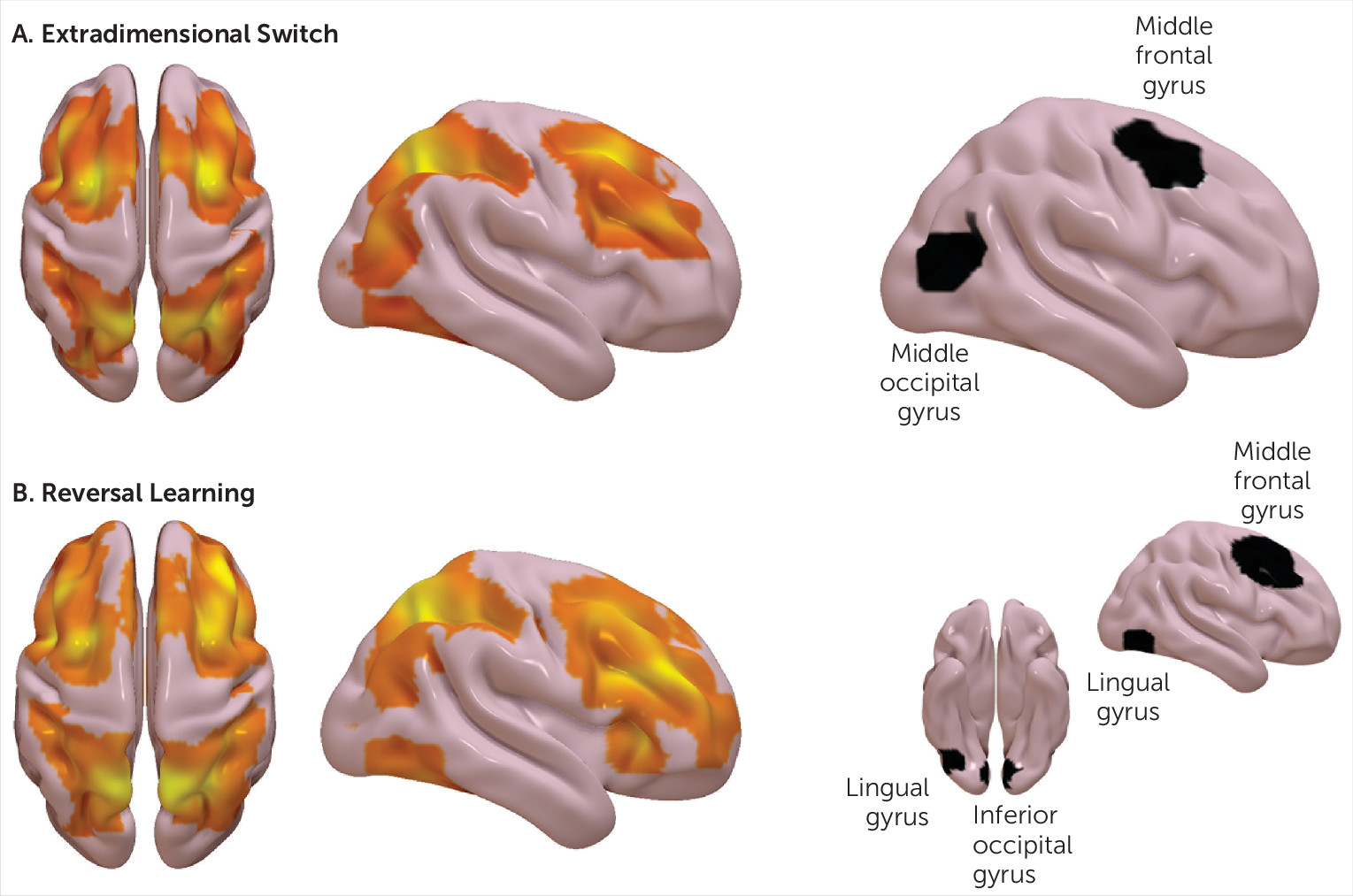
This study probed neural circuitry involved in flexible responding using a functional imaging task in patients with trichotillomania. The task activated the expected neural networks overall. Compared with controls, trichotillomania was associated with elevated activation in the right frontal gyrus coupled with reduced activation in the occipital lobe. This finding might result in improved outcomes for trichotillomania if this area proves to be a worthwhile target for treatment. Possibly due to the sample size, these group-level differences would not have withstood statistical correction for multiple comparisons, but they were with large effect size (Cohen’s d=1.2–2.0). There were no significant behavioral deficits on the task in patients, as indexed by response times or accuracy.
Because right frontal neural abnormalities (specifically, relative hyperactivation) found here in trichotillomania patients were similar for both reversal and set-shifting task contrasts, this may point to more subtle abnormalities in aspects of attentional or inhibitory control, rather than to a primary deficit in flexible responding. Previous studies have identified response inhibition impairments in trichotillomania
19 with relative sparing of set-shifting
20 and reversal learning
21 in comorbidity free cases. The right middle frontal gyrus constitutes part of a neural network involved in response inhibition, based on meta-analytic findings from functional imaging studies.
22 Interestingly, this region shows increased cortical thickness in trichotillomania compared with controls.
17 This region appears to play a greater role in adaptive online control over behavior, rather than in maintaining a task set
23, and has strong functional connectivity with other neural regions playing a primary role in suppression of triggered motor responses.
24 Previous research in OCD using the same task found hypoactivation of the lateral orbitofrontal cortices during reversal specifically.
9 Here we were not able to explore group differences in OFC activation during reversal because this region was not well represented in the activation map; this may reflect signal dropout, which is common for this region, and the relatively smaller total sample size.
In this study, we identified reduced activation of the right occipital lobe in trichotillomania patients compared with controls; geographically similar but not identical regions were affected for reversal compared with set-shifting (inferior occipital lobe for the former, middle occipital lobe for the latter). The precise role of the occipital lobe in distinct cognitive functions is not well established. This brain region is involved in the processing of visual stimuli, possibly more so for facial stimuli;
24 it is noteworthy that the current fMRI paradigm included composite stimuli, with one dimension of these stimuli being faces. The right middle occipital gyrus was significantly activated across a range of face-processing tasks, including masking and inattention tasks.
25 It would be valuable in future work to use fMRI tasks of emotional face processing in trichotillomania, in view of the aberrant activation of the occipital lobe found here across task stages, coupled with the above background literature. People with trichotillomania had lower affect regulation than controls in previous work,
26 and affect dysregulation has been suggested as a core feature of the disorder.
27Several limitations should be considered in relation to the current study. The sample size is small in this study, and therefore the study was not powered to evaluate group-level differences with statistical correction for multiple comparisons, but the findings had large effect sizes. Nonetheless, they should be regarded as being in need of replication in future work. Hair pulling severity scores were very similar for the small sample and therefore precluded an analysis of the relationship between brain activity and hair pulling severity. The study was neither designed nor powered to evaluate possible effects of treatment or gender on brain activation and cognition. All patients were women, whereas three of the controls were men; this gender balance differed significantly between the groups. Inspection of peak activation in male and female controls (see Figure S1 in the online data supplement) indicated no tangible differences; formal covariance for gender would have been inappropriate, due to the already small sample size and the fact that there were only three males.
Lastly, flexible responding is just one aspect of cognition. It would be valuable to study other domains using functional imaging in future trichotillomania research, especially response inhibition and face processing. This could be enabled by multisite collaborative research, with a view to recruiting larger samples.
There is an ongoing search in psychiatry for models of the neurobiological circuitry implicated in given disorders. One salient aspect of trichotillomania is the seemingly uncontrollable habit of pulling hair, even as the person is aware of worsening alopecia.
3 The current study found evidence for abnormal activation in the right middle frontal lobe and middle-inferior occipital cortices in patients with trichotillomania versus controls. Rather than suggesting a primary deficit in flexible responding, we suggest that this may be due to secondary deficits elsewhere, because the activation patterns were abnormal across reversal learning and set-shifting.