The capacity to regulate urges is an important human characteristic associated with a range of academic, financial, legal, physical, and mental health outcomes.
1–7 Early experiments that started in the 1960s by Walter Mischel and colleagues found that preschoolers’ ability to delay gratification was predictive of better performance based on various social, psychological, and health measures even 40 years later.
1,2 A series of follow-up studies showed that the number of seconds preschoolers were able to wait for a delayed reward correlated with higher sense of self-worth and self-esteem, better distress tolerance, fewer interpersonal difficulties, and less substance abuse.
2 In another long-term follow-up investigation, limited ability to delay gratification at age 4 was found to be associated with increased likelihood of being overweight at age 11,
5 with similar findings obtained in yet another such study.
6 Similar results have been obtained from the Dunedin Multidisciplinary Health and Development Study, in which measures of self-control in childhood predicted better health and functional outcomes in adulthood.
3,7 These findings underscore the important role of self-regulatory capacity in mental health, physical health, and psychosocial functioning.
Self-regulatory capacity has been postulated to involve limited reserve, which, when depleted, leads to failure.
8,9 An alternative explanation to this resource depletion model is that self-regulatory failure after initial effort is mediated by a shift in motivation, emotion, and attention toward more inherently rewarding action.
10,11 These models may not be mutually exclusive and may in fact be complementary. Fatigue can be seen as a cost/benefit calculation and may be caused by increased cost of continued effort, decreased value of reward, and depleted internal resources.
12 Thus, continued effort in an activity without a commensurate reward can result in an unfavorable cost/benefit evaluation and a shift in motivation toward activity that is more rewarding. Self-regulatory fatigability has been postulated as a process through which efforts to resist cravings in addiction can fail.
13 Indeed, several studies have implicated self-regulatory fatigability in substance abuse and addiction.
14–18 In addition, deficits in sustained attention, recognized as a feature of attention deficit hyperactivity disorder (ADHD),
19–21 can also be conceptualized as a form of self-regulatory fatigability.
22 Interestingly, subjective perception of mental effort during attention tasks correlates with extent of reported ADHD symptoms.
23,24 Self-regulatory fatigability may also be involved in other psychiatric conditions that are known to entail deficits in self-regulation, including Tourette’s syndrome, obsessive-compulsive disorder (OCD), behavioral addictions (pathological gambling, video game addiction, sex/pornography addiction), bulimia nervosa, and sleep deprivation. Of note, frontal control neural systems have been found to be implicated in these conditions.
The neural basis of self-regulation is believed to involve prefrontal cortical executive control, particularly the dorsal, lateral, and medial regions of the prefrontal cortex (PFC).
25,26 There have been few studies that investigated the neural basis of the phenomenon of self-regulatory fatigability. These have used a variety of different self-regulatory tasks, including emotion suppression, cognitive control, and impulse control. An early study found that white volunteers performed more poorly on the Stroop task following interaction with black individuals; the degree of worsening in Stroop performance correlated highly with racial bias scores on the implicit association test, an unobtrusive behavioral measure of racial bias.
27 The rationale is that white volunteers with higher racial bias scores required more self-regulatory effort during interaction with black individuals, which resulted in more self-regulatory fatigability during their Stroop performance. In a separate session, functional magnetic resonance imaging (fMRI) showed that activity in the right dorsolateral prefrontal cortex (DLPFC) in response to viewing black faces predicted the Stroop interference that followed the interracial interaction.
27 A later study by Inzlicht and Gutsell
27 found that after a self-control activity in which subjects were required to suppress their emotions while watching an emotional movie, subsequent performance on the Stroop task was worse compared with that of participants who watched the movie normally; moreover, electroencephalographic recordings during the Stroop task showed that the worsened performance was mediated by a weaker dorsal anterior cingulate (dACC)-associated error-related negativity signal.
28 This has been replicated more recently by another group.
29 Of interest, in the more recent replication, when subjects were instructed to regulate their emotions by reappraisal rather than suppression, there was no worsening of performance on the subsequent Stroop task and no weakening of the error-related negativity signal.
29 In an fMRI study, following an attention control task that involved having to pay attention to a film while ignoring distracting words appearing on screen, participants showed increased activity in the amygdala when shown negative emotional scenes, as well as decreased functional connectivity between the left amygdala and the ventromedial PFC.
30 The same attention control task was used by the same group with chronic dieter participants, who subsequently had greater food-cue-related activity in the orbitofrontal cortex (OFC), and decreased functional connectivity between the OFC and the inferior frontal gyrus (IFG) in comparison to chronic dieter participants not exposed to the attention control task.
31 In another fMRI study, participants who suppressed their emotions while viewing images that included negatively valenced pictures subsequently had worse performance on the Stroop task, with decreased activity in the right DLPFC compared with control participants.
32 A particularly interesting fMRI study recruited individuals who had taken part in the original preschooler gratification delay studies 40 years earlier to perform a go/no-go task; those individuals who had been identified as “high delayers” (i.e., those that were better able to delay gratification) showed significantly greater right IFG activation in the no-go versus the go trials, in comparison to the participants identified as “low delayers”
33. Finally, another, more recent fMRI study with participants from the original preschooler gratification delay studies suggested that the “high delayers” recruited a network of brain areas including IFG, ACC/superior frontal gyrus more efficiently in comparison to the “low delayers” during a working memory task.
34 Overall, studies investigating the neural basis of self-regulatory fatigability have implicated the PFC—in particular, the dorsal, lateral, and medial regions.
Here, we contribute to this emerging literature with an fMRI investigation of the neural mechanisms of the phenomenon of self-regulatory fatigability via an eye-blink inhibitory control paradigm. We hypothesized that repeated effortful eye-blink inhibition would show evidence of self-regulatory fatigability manifesting as an increase in the number of eye blinks escaping inhibitory control; that inhibition of blinking would invoke known cortical frontal control areas, as well as regions involved in the experience of urge and interoceptive processing; and that self-regulatory fatigability would involve altered activity in PFC subregions involved in self-regulatory control, including the DLPFC, IFG, and ACC, and interoceptive processing areas such as the ventral PFC and parietal cortex.
Results
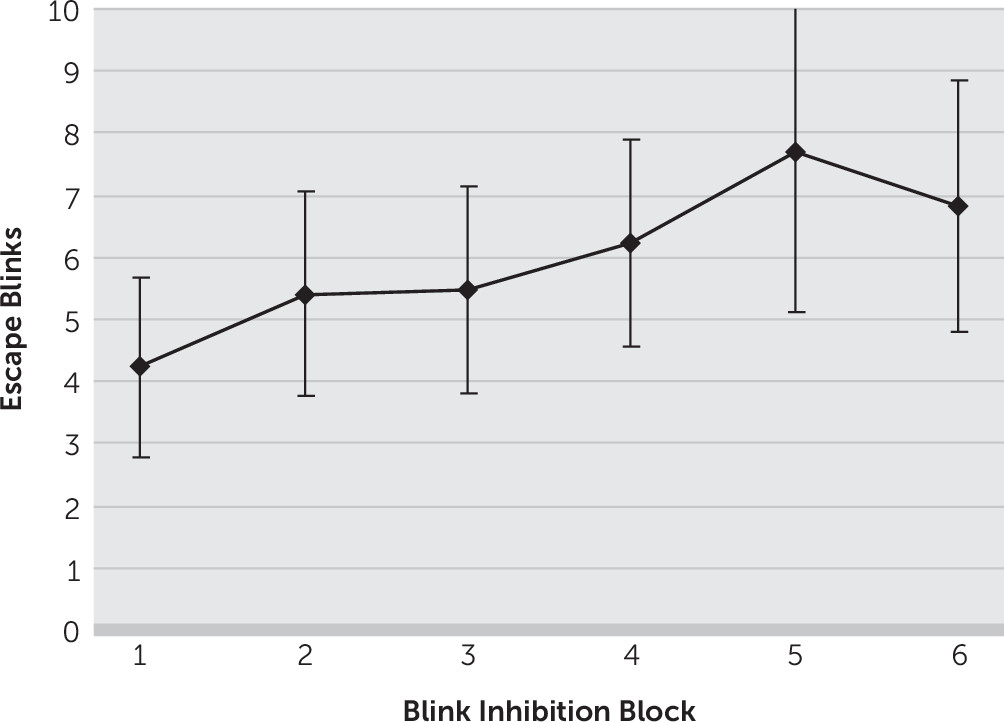
There was an increase in the number of eye blinks escaping inhibitory control across blink inhibition blocks from an average (± standard deviation) of 4.2 (±5.9) escape blinks in the first “no blink” block to 6.8 (±8.4) in the sixth “no blink” block (
Figure 1). Thus, there was an average increase of 2.6 (±3.8) escape blinks from the first to the last “no blink” blocks (p=0.01). In contrast, there was no significant change in rest blink rates from baseline, with 27.8 (±13.6) and 28.5 (±19.0) blinks in the first and last “blink” blocks, respectively (p=0.82).
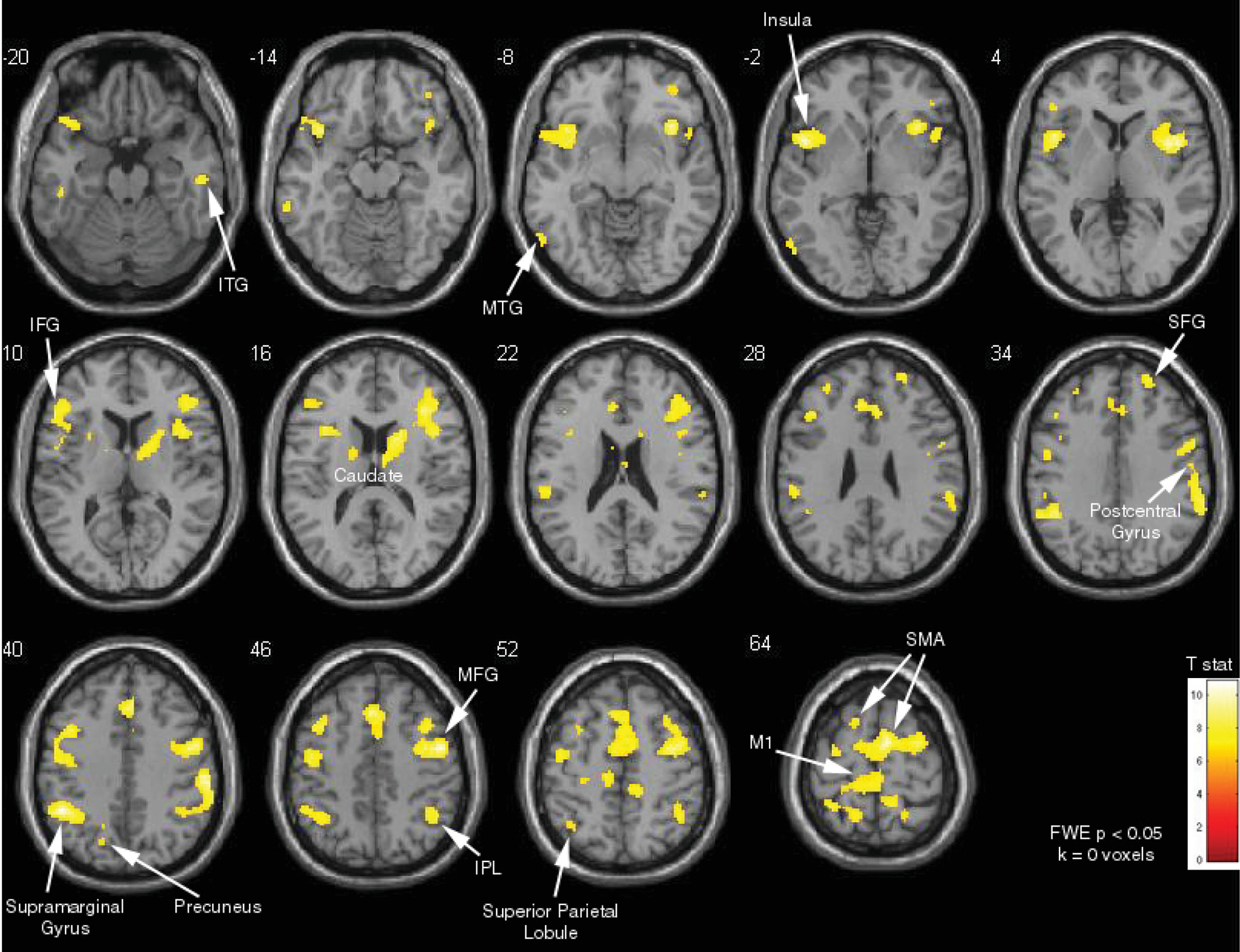
Contrasting the “no blink” and “blink” blocks revealed that inhibition of blinking activated a wide network bilaterally, including the IFG, DLPFC, dACC, frontal eye fields, supplementary motor area, and caudate (family-wise-error-corrected, p<0.05) (
Figure 2). There were also bilateral activations in the inferior parietal, anterior insula, precuneus, and sensory association areas (p<0.05, family-wise-error-corrected).
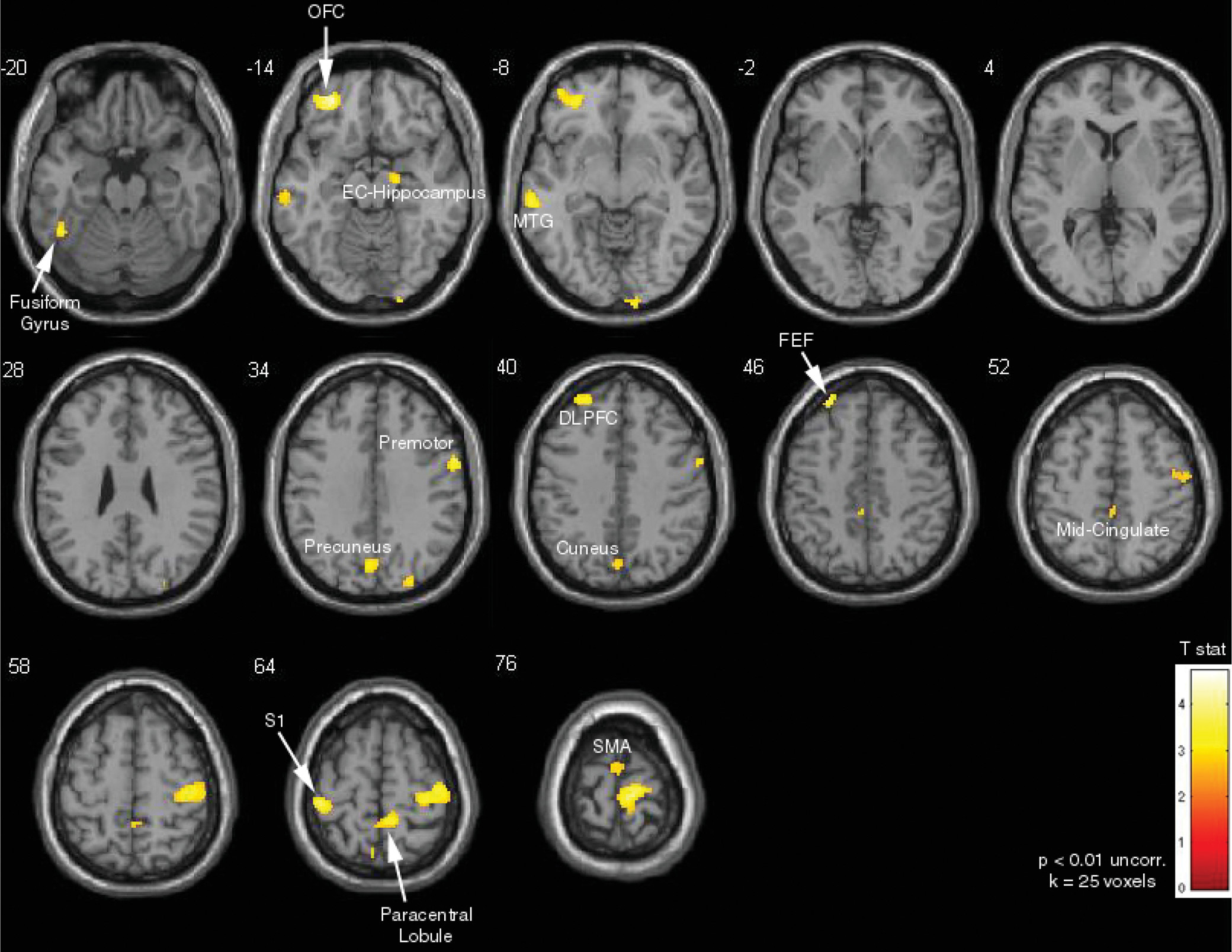
Areas positively correlated with increase in escape blinks, identified by using the increase in number of escape blinks as a covariate of interest in the “no blink” versus “blink” block contrast analysis, included the left OFC, left DLPFC, bilateral supplementary motor area, cuneus, precuneus, midcingulate, and bilateral somatosensory areas (p<0.01 uncorrected, extent thresholding k=25 voxels) (
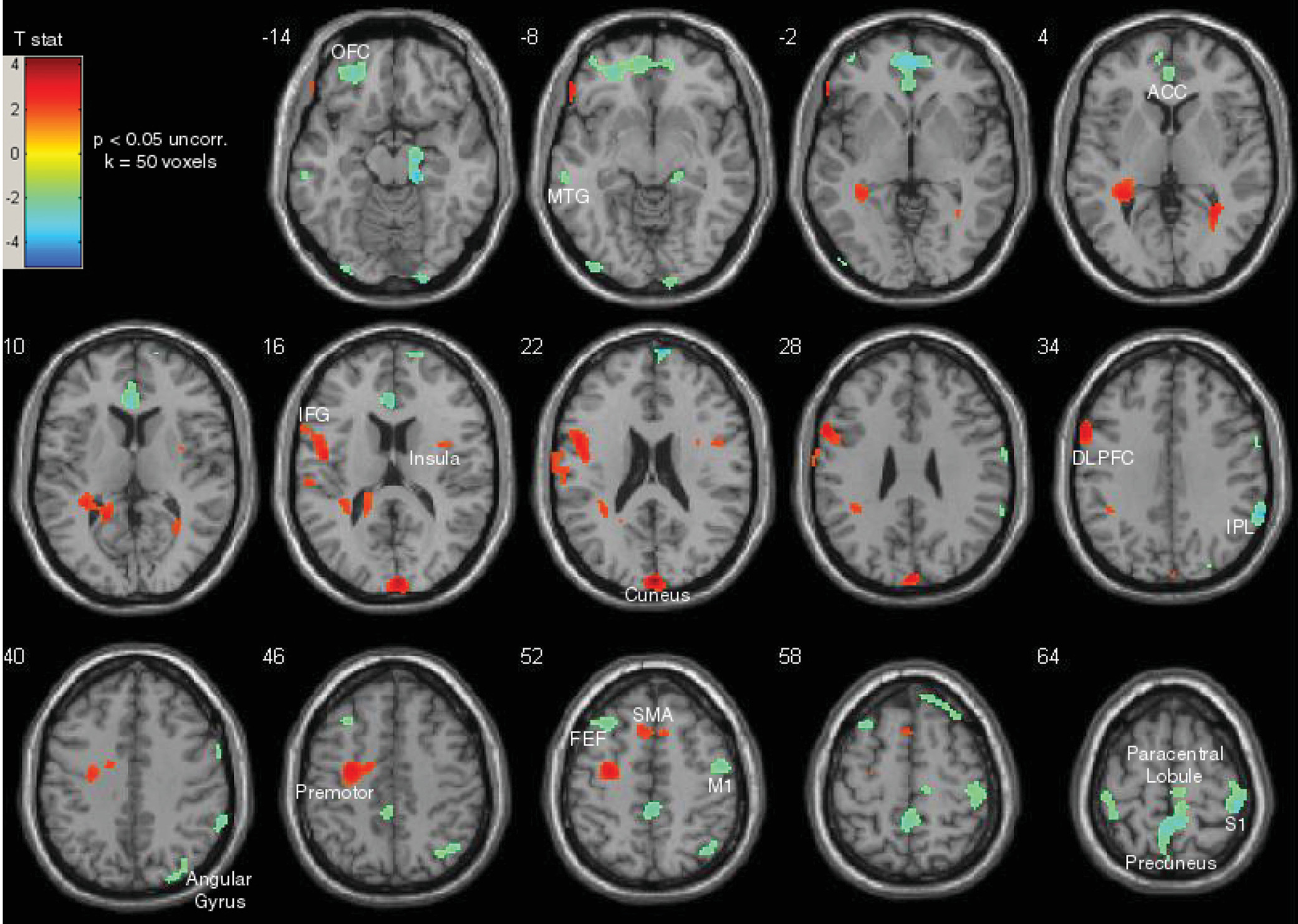
Figure 3). Finally, the top performers (i.e., those who had the least increase in escape blinks) (N=7) were contrasted with participants who had the poorest performance (i.e., those who had the most increase in escape blinks) (N=7). The top performers showed relatively higher task-related activity in frontal control areas, including the left DLPFC, left IFG, and bilateral supplementary motor areas (p<0.05 uncorrected, extent thresholding k=50 voxels) (
Figure 4). In contrast, participants who had the poorest performance had relatively higher task-related activity in the left OFC, ventral ACC, right inferior parietal lobule, and bilateral somatosensory cortex.
Discussion
In this study, we demonstrated behavioral evidence of self-regulatory fatigability in an eye-blink inhibition task. There was a progressive, significant increase in escape eye blinks across blink inhibition blocks (
Figure 1). In contrast, there was no significant change in blink rates during rest blocks. As anticipated, inhibition of blinking activated PFC control areas, including the DLPFC, IFG, dACC, supplementary motor areas, and areas involved in interoceptive processing, which included the anterior insula, inferior parietal lobule, and sensory association areas (
Figure 2). This is consistent with previous studies of self-control using suppression of bodily urges, including eye-blink inhibition,
35,36 cough suppression,
37 breath holding,
38 urinary bladder control,
39,40 and control over the urge to scratch an itch.
41 Worsening performance was associated with activity in overlapping areas, including the cuneus, precuneus, DLPFC, and SMA, as well as the OFC, midcingulate, and somatosensory areas (
Figure 3). The cuneus has been implicated in inhibitory control in persons with bipolar depression
42 and, along with the precuneus, in differential activation between pathological gamblers and control subjects in response to visual gambling cues.
43 Furthermore, given its functional connectivity to the motor cortex, dorsomedial prefrontal cortex, and DLPFC, the precuneus is believed to be involved in sensorimotor and cognitive functions related to these regions.
44 The midcingulate cortex has been involved in itch relief
41,45 and other sensory processing functions.
46,47 The OFC has been associated with subjective sensations of fatigue with prolonged performance on the Trail-Making Test
48 and craving in response to visual cues among individuals who abuse cocaine.
49 More generally, the OFC is known to have an evaluative role regarding the rewarding or aversive consequences of actions.
12 The positive correlation between escape blinks and the DLPFC, SMA and premotor areas may be a compensatory response to fatigue.
50 Contrasting the top performers with those that most demonstrated the phenomenon of self-regulatory fatigability showed a particularly interesting pattern: while the former had relatively higher activation in known PFC control areas—the IFG, DLPFC, SMA—the latter had relatively higher activation in the ventromedial PFC, rostroventral ACC, and OFC (
Figure 4), areas more related to response to hedonic impulses.
25 Self-regulatory fatigability was also associated with relatively higher activation of sensory areas and the inferior parietal lobule.
Our findings are consistent with a model of response inhibition and salience attribution that has been used to explain emotional, cognitive, and behavioral changes seen in addiction.
25 According to this model, higher-order control or “cold” processes are subserved by dorsal and lateral subregions of the PFC. On the other hand, automatic, emotion-laden, or “hot” processes, are associated with ventral subregions of the PFC. Thus, the activations in the high performers suggest that the urge to blink remains controlled by the control subregions of the PFC. In contrast, self-regulatory fatigue was associated with activations in ventral subregions of the PFC, suggesting reduced self-control, with emotion and attentional resources directed to craving. Interestingly, this group also showed relatively higher activation of somatosensory and interoceptive processing areas, including the inferior parietal lobule, which has been found to be involved in the experience of urges. In a 2-deoxy-2[18F]fluoro-
d-glucose positron emission tomography study conducted by Volkow and colleagues,
49 instructions for individuals who abuse cocaine to suppress their craving when watching a cocaine-cue video resulted in decreased activity in the rostroventral ACC, insula, and OFC, with associated activation in right inferior frontal areas. In an fMRI study, tobacco-dependent smokers who were showed cigarette cues with instructions to resist their craving demonstrated increased activity in the dorsal ACC and superior frontal gyrus and decreased activity in sensory areas.
51 A similar study by another group showed that control over craving among smokers is associated with activity in the dorsomedial PFC, DLPFC, and ventrolateral PFC and decreased activity in the OFC and subgenual cingulate.
52 Additionally, in a particularly interesting fMRI and ecological study, increased right IFG and SMA activation during a go/no-go task was associated with weakening of the link between cravings and subsequent smoking.
53 These findings overlap with results from another fMRI and ecological study that found that the IFG mediated the effect of resistance to giving in to the temptation to eat.
54On the basis of this model, when persons who are addicted to alcohol,
15 persons who abuse cocaine,
16 or persons who are smokers
18 are exposed to the relevant cues from the relevant substance, subsequent performance on self-control tasks deteriorates as “hot processes,” subserved by ventral PFC regions, become relatively more active. Of more general clinical significance, resources used for symptom suppression may result in self-regulatory fatigability and difficulty in maintaining executive control. For example, in Tourette’s syndrome, a condition known to involve multiple comorbid conditions (particularly OCD and ADHD), sustained efforts to achieve tic suppression may exacerbate difficulties with attention and affect school performance; in addition, tics and comorbid symptoms are known to be worse with fatigue, which may be a reason why tics tend to generally become worse at the end of the day.
55 In ADHD, deficits in sustained self-control
19–21 may help to explain the high comorbidity with substance abuse.
13,56 In fact, deficits in delayed gratification in ADHD
57 can be conceptualized as a form of executive fatigability. Interestingly, subjective perception of mental effort during attention tasks correlates with the extent of reported ADHD symptoms.
23,24 It therefore makes sense that environmental interventions that decrease executive demand, such as a predictable structure, planned frequent breaks, and reduction in the amount of undue stress, can be helpful in managing ADHD.
Although our study cannot settle the debate regarding the processes leading to the phenomenon of self-regulatory fatigability (i.e., the resource depletion model versus the more recent non-resource-based competing model), our findings do show distinct dorsal and lateral PFC subregions associated with consistent self-control, in contrast to the ventral PFC and other somatosensory urge-related brain areas that are associated with self-regulatory fatigability. A possible explanation, which would encompass both models as complementary, is that depletion of resources required to maintain a certain level of activity in dorsal and lateral PFC control regions results in a relative shift toward ventral PFC regions, with concomitant deterioration of performance. Accordingly, resource depletion would be accompanied by a shift in motivation, emotion, and attention toward giving in to the urge to blink. It is noteworthy that in the original studies of the delay of gratification among preschoolers, attentional control away from the immediate reward was found to play an important role in the preschoolers’ ability to maintain self-control.
1 This appears to fit with our findings, as well as with the competing model to explain the phenomenon of self-regulatory fatigability.
10 Also of interest, in the original delay-of-gratification studies, cognitive reappraisal was another successful self-control strategy.
2 This is consistent with findings from more recent neuroimaging research demonstrating that while suppression of emotions impairs subsequent executive function,
28,29,32 task performance was not adversely affected when reappraisal was used as the emotional regulation strategy.
29 In fact, distraction and environmental modification are recognized strategies for self-control in substance abuse, dieting, and daily life situations.
58 Such strategies involve activation of dorsal and lateral PFC self-control regions
59 and may function by helping to ensure that attention, motivation, and emotion resources are not directed toward hedonic, immediate-reward cues.
There are several limitations to consider here. First, although our total sample size is comparable to that of other fMRI studies, the analysis comparing the subjects on the basis of performance entailed a smaller number of subjects. It is for this reason that p-value correction was conducted via extent thresholding, as opposed to consistently via family-wise error. Another limitation was that we did not assess for motivation, nor did we have an online rating for urge and discomfort during the task. All of these are factors that could influence performance. Furthermore, we did not control for time of day, smoking, caffeine intake, or medication, all of which could affect the results, for example, through effects on arousal. Finally, our task design did not entail a longitudinal analysis that could presumably show a shift of brain area activations with self-regulatory fatigability. As such, we are unable to uncover potential compensatory changes in response to increasing fatigue.
In summary, we demonstrated behavioral evidence of self-regulatory fatigability in an eye-blink inhibition task. As anticipated, effortful eye-blink control resulted in activation of prefrontal control areas and regions involved in urge and interoceptive processing. Worsening performance was associated with activation in regions involved in affective salience attribution and craving. In contrast, high performers showed relatively more activation in prefrontal cortical control areas, including those involved in attentional control. Our findings suggest that the phenomenon of self-regulatory fatigability is associated with relatively less recruitment of prefrontal cortical control regions.
Future larger studies that include analyses of brain activation changes over time during task performance are warranted. Furthermore, investigation of changes in effective connectivity in relation to task performance could help elucidate the neural dynamics that mediate self-regulatory fatigability. Additionally, there are many opportunities for more research of self-regulatory fatigability in clinical conditions, such as substance abuse, ADHD, and Tourette’s syndrome, among others. Finally, improved knowledge in this field promises to help in the development of interventions to improve executive functions in young children,
60,61 which has the potential to have a significant positive impact on long-term outcomes.