Although antipsychotics are efficacious in the management of positive symptoms of schizophrenia (
1), a sizable proportion of patients with schizophrenia do not respond to treatment and are classified as “treatment resistant.” Published rates of treatment-resistant schizophrenia range between 20% and 60% (
2,
3). Clozapine, a second-generation antipsychotic, is the drug of choice for treatment-resistant schizophrenia because of its greater efficacy and effectiveness compared with other antipsychotics (
4–
7). Moreover, clozapine is the only antipsychotic recommended for the management of recurrent suicidal behavior (
1,
8), which affects up to 50% of patients with schizophrenia (
9,
10). Despite these recommendations, rates of clozapine use have remained low in the United States, ranging between 2% and 10% (
11,
12). Although contraindications related to clozapine’s health risks and patients’ resistance related to the mandatory blood monitoring may explain a portion of the underuse, providers’ resistance toward prescribing clozapine is a likely driver of underuse (
13–
16).
In contrast to the underuse of clozapine, the concurrent use of two or more nonclozapine antipsychotics (hereafter, antipsychotic polypharmacy) for prolonged periods is frequent, involving up to 50% of patients with schizophrenia depending on study methods and populations studied, and the practice is growing in the United States (
17,
18). This practice has been identified as an indicator of poor quality and as an overused treatment strategy because it lacks supporting evidence and it is associated with safety concerns and high direct costs (
19–
23). Although the reasons behind this prescription practice are poorly understood, some evidence suggests that antipsychotic polypharmacy is used as a substitute for clozapine in the management of schizophrenia (
24,
25).
Addressing the underuse of clozapine and the overuse of antipsychotic polypharmacy in Medicaid has the potential to improve system effectiveness, safety, and efficiency of care (
26), key aims of high-quality systems of care (
27). However, little is known about prescriber-level prevalence or the factors associated with these prescribing practices, information necessary for targeting interventions. We examined prescribers’ use of clozapine and antipsychotic polypharmacy in Pennsylvania Medicaid, the fourth largest Medicaid program in the United States by expenditures (
28). We examined variation in clozapine and antipsychotic polypharmacy practices across prescribers, the correlation between these two prescribing behaviors at the prescriber level, and the factors associated with their use among patients with schizophrenia.
Methods
Data
We obtained data from Pennsylvania’s Department of Human Services for all beneficiaries enrolled in Pennsylvania’s Medicaid program for calendar years 2010 to 2012. In each year, more than two million individuals enrolled in either the fee-for-service or managed care programs. The pharmacy claims file has information for each prescription including date of fill, days’ supply, dose, National Drug Code (NDC), and prescriber identifier. We linked the Medi-Span database to the pharmacy claims to identify antipsychotic prescriptions by NDC (
29). We used medical claims to identify patients’ diagnoses and the enrollment file to capture patients’ demographic information, eligibility type, and Medicaid payer (fee-for-service versus a specific managed care plan). We linked the National Provider Identifier (NPI) file to the provider file by NPI to obtain prescribers’ sex and specialty (
30).
Study Sample
For each year, we identified prescribers writing at least one antipsychotic prescription for Medicaid patients with schizophrenia ages 18–64 years and not dually eligible for Medicare. Schizophrenia was defined as at least one inpatient or at least two outpatient claims with a primary or secondary diagnosis of schizophrenia (ICD-9 code 295.XX). We then limited our study sample to providers prescribing antipsychotics to ten or more patients with schizophrenia in a single year. An individual prescriber can meet this criterion in multiple years [A flow chart of the study sample is available as an online supplement to this article.]
Outcome Variables
Main outcomes were two prescriber-level variables: annual proportion of patients with schizophrenia with any clozapine use and annual proportion of those with any antipsychotic polypharmacy. Antipsychotic polypharmacy was defined as ≥90 days’ concurrent use of at least two different nonclozapine antipsychotics, allowing for gaps of up to 32 days in days’ supply for the same medication. This definition has high specificity and positive predictive value (
31). In other words, we used information on medication ingredient name, dates of prescription fills, and days’ supply in order to measure the overlap in patient possession of two different antipsychotic ingredients for ≥90 days. Oral and depot formulations for the same drug were considered to be the same drug. We computed days’ supply for long-acting injectable antipsychotics on the basis of recommended dosing intervals (
21). In the descriptive analyses below, we also reported any use of clozapine or antipsychotic polypharmacy by prescribers in a year as secondary outcomes.
Explanatory Variables
We constructed several explanatory variables measuring characteristics of prescribers’ patient caseloads, prescriber characteristics, and indicators for Medicaid payer in a year. For each prescriber, we calculated the percentage of his or her patients with schizophrenia that were female, Hispanic, and non-Hispanic black, as well as the mean patient age. To characterize patient caseloads’ health status, we calculated prescribers’ percentage of patients eligible for Medicaid through Supplemental Security Income (SSI), a measure of disability; percentage of patients with several psychiatric comorbidities (see
online supplement); and percentage of patients with any schizophrenia-related hospitalization; we also calculated the mean number of general medical comorbidities for patients (based on the 25 non–mental health conditions in the Elixhauser comorbidity index) (
32).
We adjusted for provider sex and also measured each prescriber’s antipsychotic prescribing volume—the total number of antipsychotic prescriptions written for patients with schizophrenia annually—and classified prescribers as being low versus high volume, split by the median value. The total count of prescriptions attributed to prescribers in our study cohort was based on the total number of unique combinations of patient, ingredient, and date of service (date prescription was filled) for each prescriber. A patient’s prescriptions for the same antipsychotic medication contributed to the antipsychotic prescribing volume measure only if they were filled on different dates. In other words, prescriptions filled on the same date for the same patient had to be for different antipsychotic medications in order to contribute to the prescribing volume. We created three prescriber specialty categories: psychiatrist (most of the sample), primary care provider, and other (other physicians and non-M.D. providers). We also included year indicators to control for potential time trends.
During our study period, Pennsylvania Medicaid ran a fee-for-service program for approximately 25% to 30% of enrollees and contracted with multiple managed care organizations (MCOs) to manage care for the remaining 70% to 75%. The fee-for-service program and each of the individual MCOs implemented different utilization management tools to manage antipsychotic use, including prior authorization policies for specific populations (for example, children younger than 12 years) or for select antipsychotic medications (for example, low-dose quetiapine). Because the use of utilization management tools varied between the fee-for-service and managed care programs, as well as among MCOs, we characterized prescribers’ caseloads based on payer mix in two ways. First, we created a continuous measure of the number of managed care plans in which the provider’s patients were enrolled. Second, we created a categorical variable indicating which MCO accounted for the greatest percentage of the provider’s patients, with providers who primarily treated fee-for-service enrollees as the reference group.
Statistical Analysis
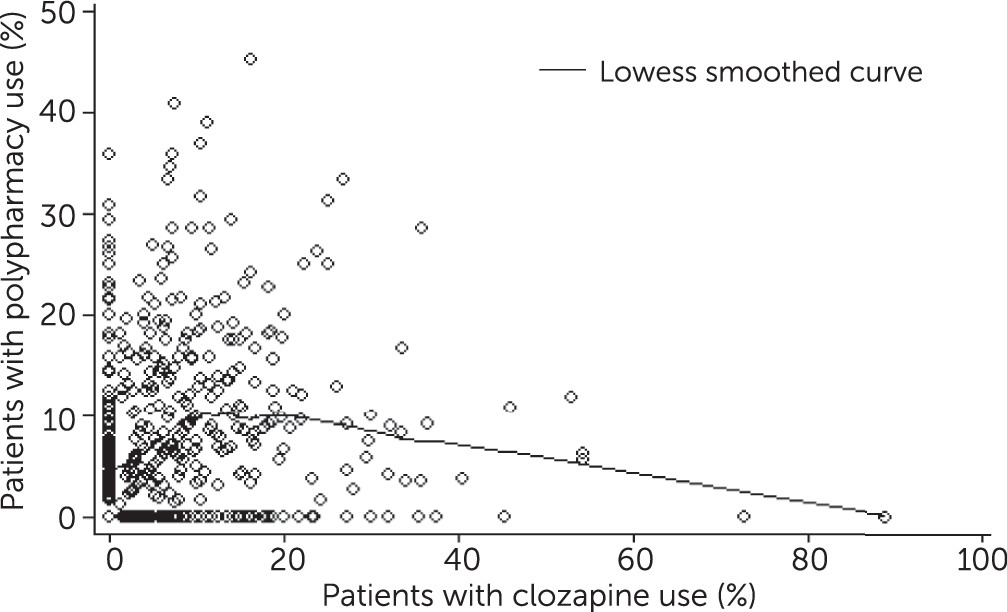
For each year, we reported prescriber-level prevalence of clozapine and antipsychotic polypharmacy prescribing, overall and stratified by prescription volume and specialty. To examine the association between prescriber-level clozapine and antipsychotic polypharmacy practices, we created scatter plots as well as the Lowess smoothed curve of the two outcomes. To account for variable patient volume across providers, we calculated a weighted Spearman’s rank correlation coefficient (rho) with patient volume as analytic weight to examine the correlation between the two prescribing measures.
We used generalized linear mixed models to examine patient caseload and prescriber characteristics associated with prescriber’s clozapine and antipsychotic polypharmacy practices. We used a binomial distribution with a logit link to handle the two variables specified as a proportion (
33). A random prescriber term was included to adjust for clustering and variable patient volume across prescribers.
We performed several sensitivity analyses. First, we conducted an analysis restricting the sample to psychiatrists, the specialists most likely to treat schizophrenia in the United States. Second, to evaluate whether the use of low-dose quetiapine, frequently prescribed as a sleep aid, may contribute to the overall rate of antipsychotic polypharmacy captured in our study, we repeated our planned antipsychotic polypharmacy analyses after excluding low-dose (<150 mg) quetiapine prescriptions. The results for the above alternative specifications were very similar to the primary analyses, and thus they are not reported. Third, because we were unable to identify patients with treatment-resistant schizophrenia or recurrent suicidal behavior in order to directly evaluate the impact of illness severity on prescribing behavior, we performed a sensitivity analysis that used schizophrenia-related hospitalization as a proxy for illness severity. We then examined variation in the two outcomes stratified by the percentage of prescribers’ patients with schizophrenia-related hospitalization.
Discussion
To our knowledge, ours is the first provider-level analysis of both clozapine and antipsychotic polypharmacy prescribing in the United States. Although clozapine’s superior effectiveness for patients with treatment-resistant schizophrenia and recurrent suicidal behavior is well established, long-term nonclozapine antipsychotic polypharmacy is a costly practice lacking empirical support. Yet we found that prescribers were as likely to use antipsychotic polypharmacy as clozapine in their treatment of patients with schizophrenia. We did not confirm our hypothesis that prescribers who used clozapine would be less likely to prescribe antipsychotic polypharmacy. In fact, the two prescribing behaviors were positively correlated at the provider level. Although patients taking clozapine or antipsychotic polypharmacy made up a low share of these prescribers’ caseloads, on average, there was considerable variation in these prescribing practices across prescribers. This analysis underscores the need for new approaches to promote evidence-based antipsychotic prescribing to improve outcomes for this population.
Although the optimal rate of prescribing clozapine is unknown, the prevalence of treatment-resistant schizophrenia and recurrent suicidal behavior—two indications for which clozapine is the only recommended antipsychotic—may be conservatively approximated to 30% (
11). Our prescriber sample managed many patients with severe illness, as evidenced by the fact that on average 43% of their patients had a schizophrenia-related hospitalization. Although we were not able to directly measure the prevalence of treatment-resistant schizophrenia in our data, the low prevalence of clozapine prescribing in our sample (only 7% of the patients) is a potential signal of underuse of clozapine, a persistent quality concern in schizophrenia care (
34). Prescribers’ reluctance to prescribe clozapine might stem from concerns over its safety (for example, agranulocytosis and metabolic risk), lack of knowledge or experience, or higher administrative burden associated with the monitoring program (
13–
15,
35). Although prescribers ought to weigh safety concerns against the potential benefit of any drug, prescribers tend to overestimate the prevalence of side effects and risks associated with clozapine (
14,
34) and may not be aware that the incidence and lethality of agranulocytosis have been dramatically reduced by the monitoring program (
36). It is unlikely that concerns about clozapine’s metabolic risk are a main driver of its underuse given the higher use of other antipsychotics with substantial metabolic risk and the low use of metabolic monitoring, a recommended practice aimed at reducing risk associated with use of second-generation antipsychotics (
37,
38).
It is of concern that the typical prescriber in our sample used antipsychotic polypharmacy as much as clozapine (for roughly 7% of his or her patients with schizophrenia) and that roughly one in six prescribed antipsychotic polypharmacy but not clozapine. When faced with poor treatment response to antipsychotic monotherapy, prescribers’ reluctance to switch to a new drug may be an important driver of antipsychotic polypharmacy (
24,
39). Although clozapine is the only recommended antipsychotic for treatment-resistant schizophrenia and recurrent suicidal behavior, there is evidence that prescribers use antipsychotic polypharmacy in lieu of clozapine to manage this population (
24,
25). However, we did not find evidence that antipsychotic polypharmacy was used in place of clozapine; in fact, the overall correlation coefficient of these practices was positive and statistically significant. Although a prescriber’s use of clozapine suggests a willingness to adhere to evidence-based prescribing practices, our findings indicate that at least some clozapine prescribers do not shy away from non–evidence-based practices, such as antipsychotic polypharmacy.
Our analyses of factors associated with prescribers’ using clozapine and antipsychotic polypharmacy generated several findings with important implications. First, compared with low-volume prescribers, high-volume prescribers had a larger share of patients with any use of both clozapine and antipsychotic polypharmacy, indicating that volume of patients with schizophrenia is inconsistently associated with evidence-based prescribing. Second, our finding that prescribers with the highest proportion of patients with schizophrenia-related hospitalization were much less likely to prescribe antipsychotic polypharmacy than their counterparts is suggestive of a possible impact of the growing scrutiny of this practice by the Joint Commission (JCAHO) since the late 2000s (
40). Third, we adjusted for the potential effect of patient case mix by including a rich set of patient-level demographic characteristics and comorbidities. Although we found a significant relationship between patient case mix and prescribers’ clozapine and antipsychotic polypharmacy practices, the effects were relatively small. Furthermore, the substantial variation in clozapine and antipsychotic polypharmacy practices across managed care plans suggests that differences in plans’ formularies and utilization management may have important implications for the quality of schizophrenia care.
State Medicaid programs, their managed care contractors, and health care organizations may consider a number of strategies to increase clozapine use for patients with a favorable risk-benefit profile and reduce antipsychotic polypharmacy. Specifically, quality improvement strategies—such as academic detailing aimed at improving prescribers’ knowledge of the effectiveness and risks of both practices, as well as auditing and providing feedback to prescribers who are more likely to prescribe antipsychotic polypharmacy—have the potential to increase evidence-based antipsychotic prescribing practices (
11,
13,
15). Another potential strategy is the use of financial incentives, such as pay-for-performance coupled with indicators measuring quality of antipsychotic prescribing. Rewarding prescribers for high prescribing quality might influence their behavior and increase rates of evidence-based prescribing. In addition, Medicaid and their contractors may consider utilization management tools, including prior authorization and step therapy, to increase clozapine use when clinically appropriate and discourage antipsychotic polypharmacy practices in the absence of a clear clinical rationale. Targeting efforts to high-volume prescribers and frequent prescribers of antipsychotic polypharmacy may be an efficient way to implement these policies given their larger population reach.
Our study had several limitations. First, we examined prescribers’ clozapine and antipsychotic polypharmacy practices in the Pennsylvania Medicaid program, and thus the findings may not necessarily be generalizable to other states. Second, our data source does not allow for a precise estimation of the prescriber-level annual percentage of patients with treatment-resistant schizophrenia or suicidal behavior for a given prescriber. Our focus on prescribers who have caseloads of multiple patients with schizophrenia treated with antipsychotics and who have patients with high rates of hospitalization and disability suggest that most, if not all, of the prescribers in our sample treated at least some patients with treatment-resistant schizophrenia or suicidal behavior. Nevertheless, we cannot rule out the possibility that differences in prescribing practices are driven by differences in case mix, especially prevalence of treatment-resistant schizophrenia or suicidal behavior. Third, we could not rule out the potential effect of patient preferences on prescribers’ clozapine and antipsychotic polypharmacy practices. Finally, we had a limited number of provider-level characteristics (specialty, sex, and prescription volume) to explain variation in clozapine and antipsychotic polypharmacy prescribing. Other factors such as prescribers’ age and education background might also play a role in prescribing behavior.