Measures of functional status, or the capacity to perform tasks and activities, have been increasingly the focus of medical and psychiatric outcome research. Among psychiatric conditions, syndromal and subsyndromal depression have been found to be significantly associated with long-lasting decrements in functioning that are comparable to, or even greater than, those associated with chronic medical illnesses.
1 Furthermore, when depressive disorders and chronic medical conditions coexist they appear to have unique and additive effects on functioning.
2Depressive disorders and diminished functional capabilities have been recognized as common consequences of stroke. Previous investigators have demonstrated that poststroke depression during the acute phase of recovery adversely affects functional abilities,
3–5 short-term physical therapy outcome,
4 and long-term functional recovery.
6,7 Hence, studying the impact of depression on functioning of stroke patients is important for several reasons. The magnitude of the public health problem and the economic burden imposed by stroke are considerable. Epidemiological studies demonstrate that cerebrovascular disease is the second leading cause of long-term disability among elderly people, with an annual incidence of 400,000 in the United States of America.
8 The aggregate lifetime cost for all strokes that occurred in the United States in 1990 was estimated to be 38.7 billion dollars.
9 The cost associated with rehabilitation accounts for 52% of the total cost per stroke patient.
10 Although 20% to 50% of stroke patients may have demonstrable depression,
11 a significant proportion of these depressions go unrecognized and untreated,
12 implying that the negative impact of depression on functional impairment is a major concern for health care services. A better understanding of the relationship of depression and stroke-related functional impairment may enhance awareness among clinicians and researchers of the importance of evaluating depression in the assessment and treatment of functional disabilities in stroke patients.
Previous reports concerning poststroke depression and functional impairment have yielded inconsistent results. Some studies have shown a significant association between measures of depression and functional impairment during the initial period following stroke.
3–5,13,14 Some studies found that this relationship decreased with time after stroke,
3,12 while other studies reported that this correlation increased with time.
15 Furthermore, although some studies have failed to find any correlation between depression and functional impairment during the initial period after stroke,
16,17 others have demonstrated a positive correlation at only 12 and 24 months after stroke.
18 Interestingly, Parikh et al.
6 reported that depressive measures immediately following stroke predicted follow-up functional measures at 2 years post stroke. Thus, the correlation between depression and functional impairment, and the relationship of such impairment with time since stroke, appear complex.
The relationship between functional impairment and poststroke depression is poorly understood at the present time. Some people have argued that stroke patients become depressed as a psychological reaction to limited physical functioning;
19 others speculate that depression might produce functional impairment as a result of fatigue, hopelessness, and diminished motivation;
14 and a third point of view is that physical disabilities can be both a cause and an effect of depression.
13 However, discrepancies in the findings of cross-sectional and longitudinal studies currently prevent us from reaching any firm conclusions about the nature of this relationship. Variations in sample characteristics
17 and size,
14 the time since the stroke,
5 the type of assessment instruments used, and the type of statistical analysis
4 used may have contributed to these conflicting findings. Further, although several medical, neurological, and social demographic variables might influence poststroke functioning, none of the previous studies examined the effect of these variables on the association between poststroke depression and functional impairment. Hence, it is possible that functional impairment attributed to poststroke depression could have been partly due to the confounding effects of these factors.
The Stroke Data Bank (SDB) is a multicenter, prospectively collected large pool of information from a wide spectrum of stroke patients that may serve as a resource to answer some of the unresolved research issues in cerebrovascular disease.
20 In this article, we use SDB findings to examine the relationship between functional impairment and depression after stroke. Given that initial functional scores may be critical in determining the outcome of rehabilitation of stroke patients,
21 we examined whether depression immediately following stroke would be independently associated with limitations in physical functioning. Further, we attempted to identify the physical illnesses and demographic factors that might affect functional status during the acute period after stroke.
METHODS
Subjects
Complete details on the organization and design of the Stroke Data Bank study and data collection have appeared elsewhere.
22 The four participating centers included the University of Maryland Hospital, Baltimore; Boston University Medical Hospital, Boston; Michael Reese Hospital and Medical Center of Chicago; and Columbia Presbyterian Hospital, New York. Patients with a definable stroke (stroke was defined as a sudden, nonconvulsive, focal neurological deficit persisting beyond 24 hours) who were 15 years of age or older were eligible to participate in the study. Patients were ineligible for the study if they had a coexisting, confounding physical disorder (cancer, sickle cell disease, other hematological disease, or head injury), if they were discharged from the hospital before completing the initial assessment, or if they (or, in the case of incompetent patients, the legal next of kin) did not give informed consent to participate.
Evaluation of Patients
Enrolled patients were evaluated on several parameters by a designated neurologist and research assistant in each of the five participating centers. For the purpose of the current study, data obtained 7–10 days after stroke that pertained to sociodemographic characteristics, depression, neurological and medical status, functional evaluation, and CT scan findings were used for analyses.
Functional Assessment:
Activities of daily living (ADL) assessment was performed by using the Barthel Index (BI)
23 to evaluate the functional capabilities of stroke patients. The BI is a weighted instrument that comprises 10 items measuring performance in self care (feeding, bathing, perineal care, toileting, dressing, bowel and bladder care) and mobility (transfer, ambulating, and stair climbing). The total score ranges from 0–100, with 100 representing functional competence in all 10 items and 0 representing total dependence. Functional scores were obtained by a research nurse by patients' demonstrating or reporting on the level at which each activity can be accomplished. The BI has been widely used as a functional assessment scale in stroke populations. It is simple to use and has good interrater reliability, internal consistency (α=0.96), and construct and criterion validity.
21,24–27Depression:
Depression was assessed by using the Center for Epidemiological Studies Depression (CES-D) Scale.
28 This self-report instrument consists of 20 items, of which 16 represent a variety of affective, cognitive, behavioral, and somatic symptoms associated with depression. Four items that focus on positive moods have reversed scoring. Each item is scored from 0 to 3, depending on the frequency or duration with which a specific symptom has occurred during the preceding week. Thus, for any individual, the total CES-D score can range from 0 to 60, with higher scores indicating greater severity of depression. In stroke populations, a score of 16 or higher has been found to have a specificity of 0.90, a sensitivity of 0.86, and a predictive validity of 0.80 when compared with a psychiatric diagnosis of major depression.
29 Further, when the CES-D is administered by a research nurse to stroke patients, it has been found to have good interrater reliability and good concurrent, discriminant, and construct validity.
30A research nurse read each question of the CES-D scale to the patients, who were then asked to choose the card that best described their feelings or behavior during the past week. This scale was not administered to sedated, aphasic, non–English speaking, comatose, or uncooperative patients. Stroke patients who scored 16 or higher were considered to be depressed, and, based on previous findings,
30 a score of 25 or greater was considered indicative of severe depression.
Neurological Evaluation:
Neurological examinations were conducted by neurologists, and the findings were categorized into three groups for the purpose of analysis. Motor loss was quantified by using a weakness scale (summated scores could range from 0 to 60.12), and sensory impairment was assessed by using a sensory scale (summated scores could range from 0 to 36.
12,22). Other neurological findings, including cranial nerve dysfunction, aphasia, apraxia, aprosodia, agnosia, neglect, and involuntary movements, were grouped under the heading “neurological deficits.” For the purpose of this analysis, we scored only the total number of “neurological deficits.” The diagnosis of dementia was based on the clinical judgment of the examiner. CT scans were examined by a designated CT reader (radiologist), and the information was recorded by a nurse coordinator. The following CT scan data were used in our analyses: the number, laterality, location, and size of vascular lesions and the degree of cortical atrophy.
Medical Illnesses:
Medical illnesses were assessed on the basis of history, physical examination, and relevant investigations. The following medical conditions were assessed for their impact on patient functioning: myocardial infarction, valvular heart disease, congestive heart failure, atrial fibrillation, hypertension, chronic pulmonary disease, and diabetes mellitus.
Prestroke Functioning:
Information on prestroke functioning, including eating, dressing, bladder and bowel functions, domestic activities, leisure and social activities, exercise, sleeping pattern, sexual behavior, energy level, appetite, and social network were collected for comparison with poststroke functioning. This information was collected from the patient, but if the patient was unable to remember or respond, the relevant data were obtained from the family, significant others, or staff members of the institution.
Statistical Analysis
Intergroup comparisons (depressed versus nondepressed) of categorical variables were performed with χ2 tests, and continuous variables with t-tests (two-tailed). Linear regression analysis was used to examine the relationship between BI scores and the various measures. In the regression analysis, BI scores of all stroke patients formed the dependent variable; the independent variables were depressive disorder (CES-D≥16), age, neurological variables (weakness scores, sensory scores, and other neurological deficits), clinical diagnosis of dementia, lesion volume, confounding medical illnesses, medications, prestroke functioning, and sociodemographic factors (gender and race). Linear regression analysis was also used to assess the interactions of weakness scores by depressed group. The significance of association between severity of depressive symptoms and functional impairment was examined by using Pearson's correlational analysis. All statistical analyses were conducted by using the SAS computer program. Bonferroni corrections for multiple comparisons were not undertaken because we present the actual level of significance for all comparisons and comment on results significant at the P≤0.05 level.
RESULTS
Sample Characteristics
Of the 1,806 stroke subjects entered in the SDB, 626 completed the CES-D. Of these 626 CES-D completers, 160 scored 16 or higher on this instrument and constituted the depression group. Depressed patients were initially divided into 80 with severe depression (CES-D≥25) and 80 with moderate depression (CES-D of 16–24). However, since these two groups did not significantly differ in functional impairment (
t=1.01, df=155,
P=0.31) or in demographic characteristics, they were combined into one depressed group for further analyses. The demographic characteristics of the depressed and nondepressed group are shown in
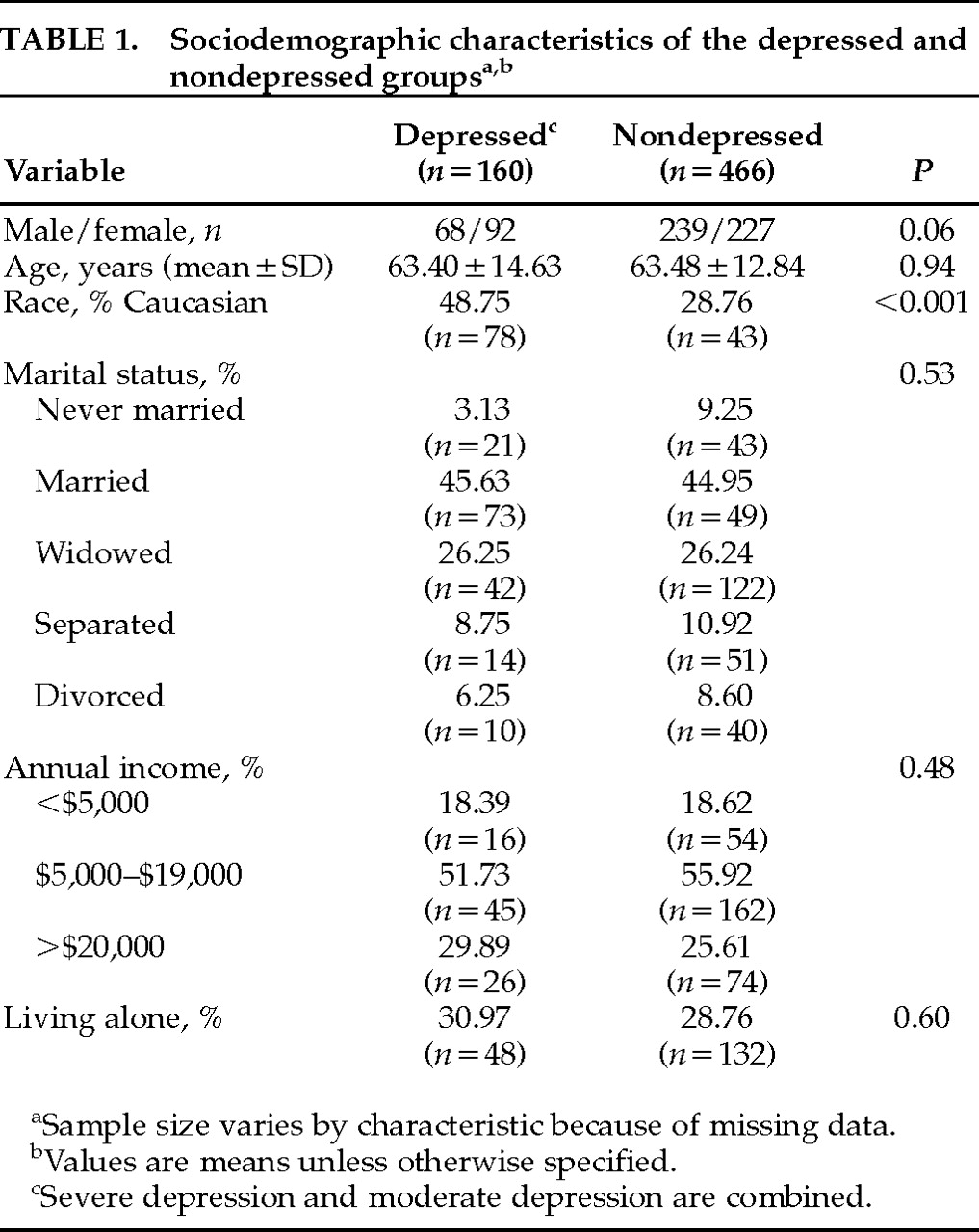
Table 1. Whites were more likely to be depressed than other ethnic groups. There was a trend suggesting that women with stroke might be more frequently depressed than men. There were no intergroup differences for total household income, marital status, or age. It is of interest to note that only 15% of depressed stroke patients were clinically identified as depressed by physicians and only 10% of depressed patients received antidepressants.
Functional Measures and Depression
Patients with depression had significantly more impaired functioning than nondepressed patients (
t=5.10, df=624,
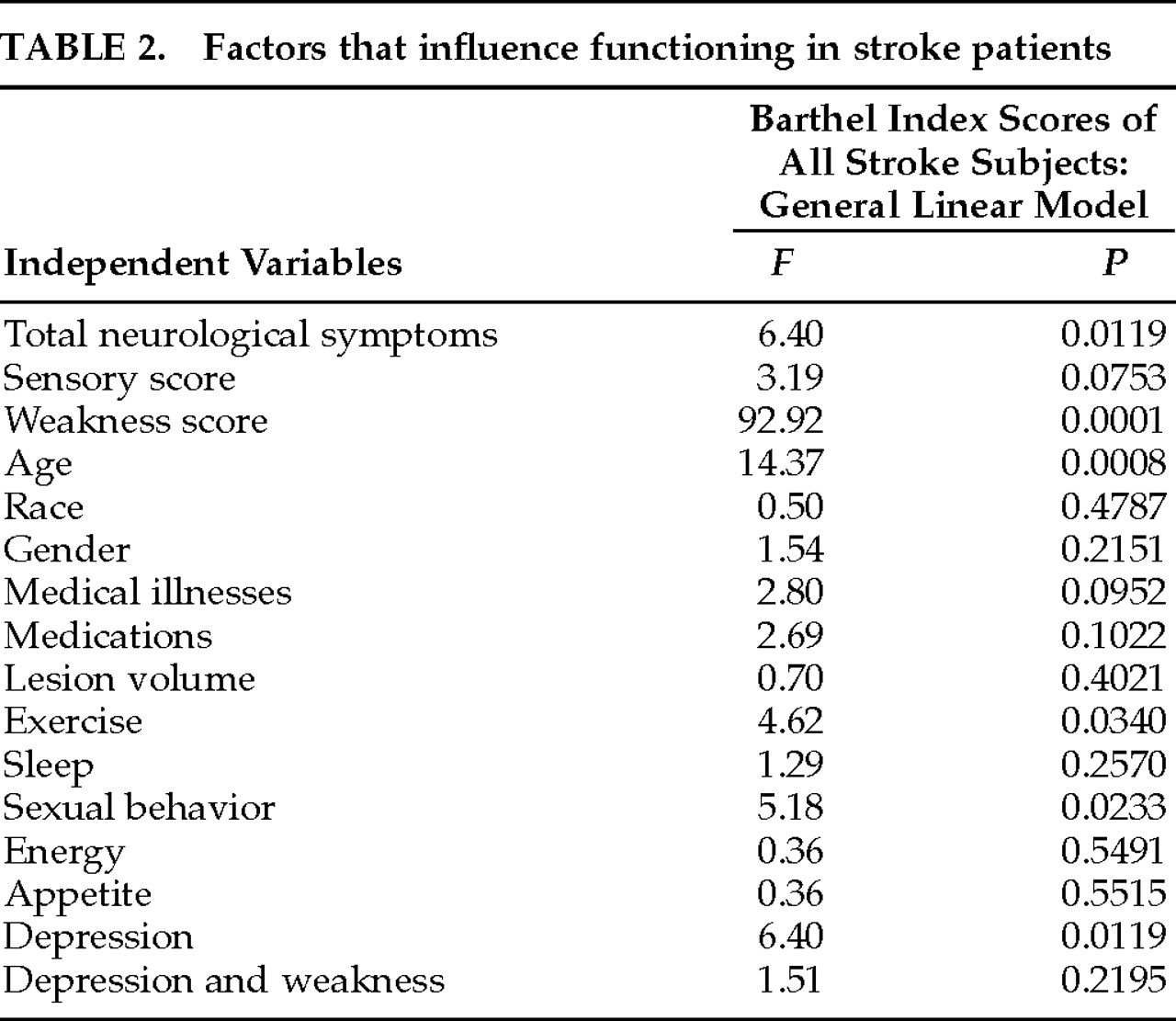
P<0.0001). Multiple regression analysis revealed that depression was independently associated with BI scores of all stroke patients (
Table 2). For the entire stroke population, scores on the CES-D scale were negatively correlated with Barthel scores (
r=–0.25,
P=0.0001). Thus, greater functional impairment was associated with higher depression scores.
To determine whether ADL impairment in depressed stroke patients was related to specific depressive symptoms, we examined the correlation between specific depressive factors and the total BI score of depressed stroke patients. Previous work with the CES-D in community samples has consistently identified four factors: depressive affect, positive affect, interpersonal relations, and somatic/retardation factors.
31 In order to determine the impact of retardation on functioning in depressed stroke patients, we analyzed the somatic and retardation factors separately. None of the five factors significantly correlated with the BI scores of the depressed group (depressed affect:
r=−0.02,
P=0.75; retardation:
r=−0.13,
P=0.08; somatic:
r=0.01,
P=0.83; positive affect:
r=0.13,
P=0.10; interpersonal:
r=0.04,
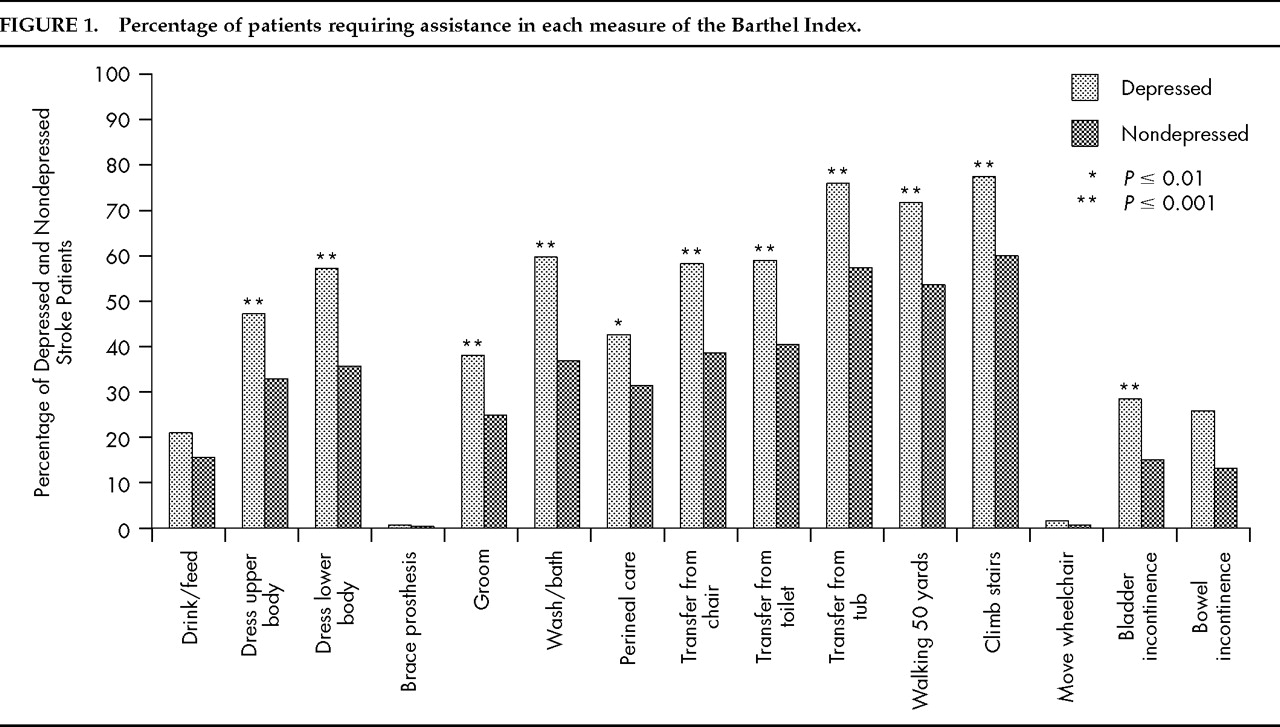
P=0.58). Each item of the BI was then examined for its relationship to depression. Compared with the nondepressed group, a significant proportion of depressed stroke patients required assistance in performing tasks in almost all functional items except drinking and feeding, brace prosthesis, and moving the wheelchair (
Figure 1). This finding suggests that depression-related functional impairment involves both self care and ambulation.
Although women evidenced greater functional impairment than men (
t=−2.54, df=624,
P=0.01), the general linear model did not show a significant interaction of gender and depression with respect to functional impairment (
F=74.08, df=1,
P=0.75). Similarly, although age was negatively correlated with BI scores (
r=−0.18,
P=0.0001), there was no age by depression effect (
F=21.31, df=1,
P=0.86). Depressed subjects had a higher rate of myocardial infarction (χ
2=8.10, df=1,
P=0.04), aortic stenosis (χ
2=4.92, df=1,
P=0.02), and mitral prolapse (χ
2=8.75, df=1,
P=0.003) than nondepressed stroke patients. Also, depressed patients were more frequently prescribed steroids (χ
2=13.88, df=1,
P=0.000), narcotics (χ
2=16.55, df=1,
P=0.000), anticonvulsants (χ
2=5.15, df=1,
P=0.02), insulin (χ
2=3.78, df=1,
P=0.05), and antidepressants (χ
2=10.48, df=1,
P=0.001) during hospitalization. However, on regression analysis, the confounding medical illnesses and medication history did not independently correlate with functional impairment of stroke patients (
Table 2).
Neurological and CT Scan Variables
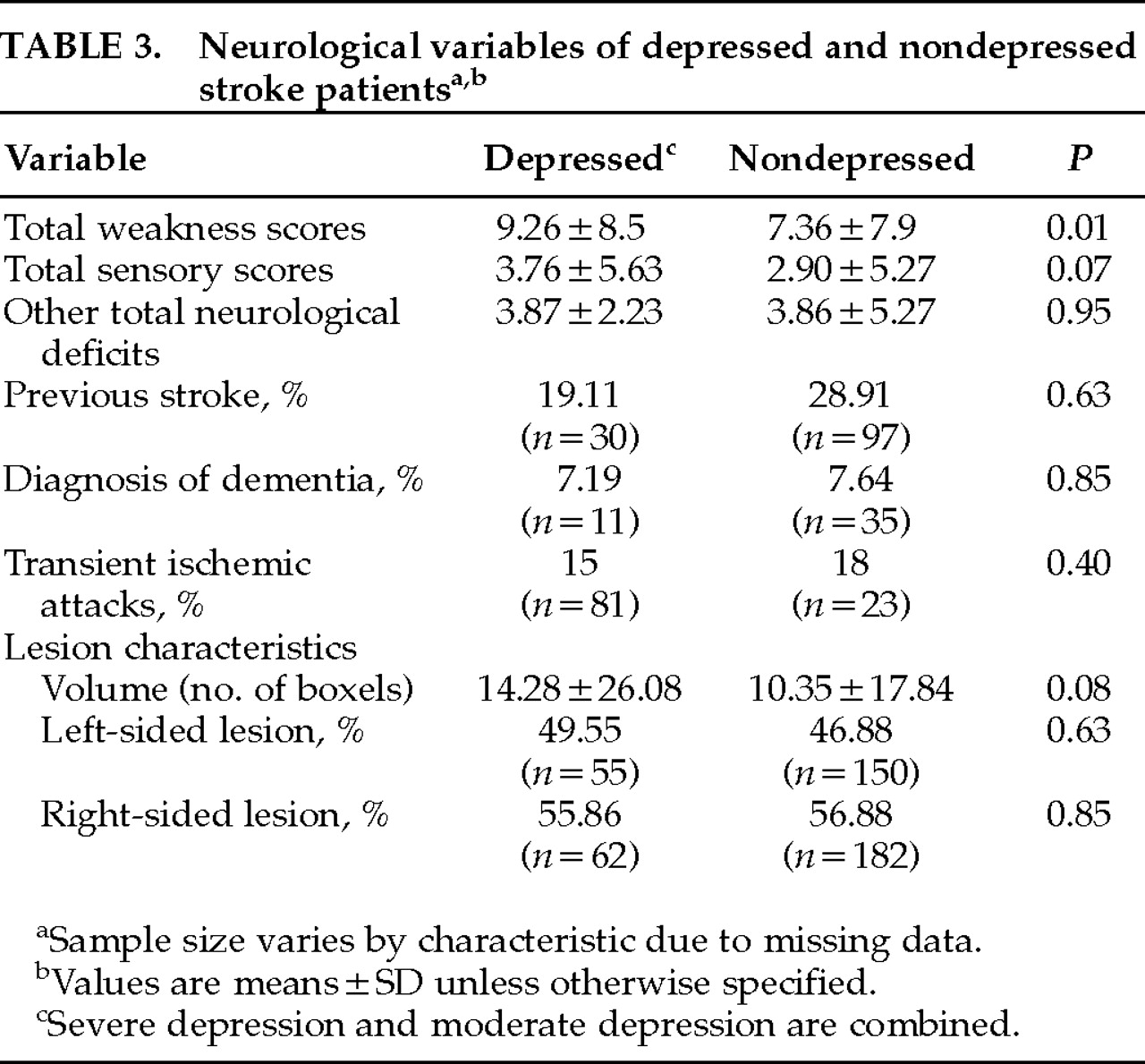
There were no statistically significant differences between depressed and nondepressed groups in diagnostic subtypes of stroke (χ
2=10.42, df=7,
P=0.16), previous history of stroke, and transient ischemic attacks (
Table 3). Multiple regression analysis revealed that weakness scores and total neurological deficits were independently associated with functional impairment in stroke subjects (
Table 2). Although total weakness scores were significantly higher in depressed than in nondepressed stroke patients (
Table 3), when weakness scores were controlled, depression remained a statistically significant factor that affected function (
F=20.55, df=1,
P=0.0001). There were no differences between depressed and nondepressed patients in lesion characteristics (laterality and volume;
Table 3). It should be noted, however, that a previous study demonstrated that among SDB subjects with single lesions less than 7 boxels in size, depression was significantly associated with left hemisphere injury.
32 In the present study, regression analysis did not show a relationship between lesion volume and BI scores in stroke subjects (
Table 2). Thus, characteristics of brain lesions could not account for the observed differences in functional impairment between depressed and nondepressed.
Prestroke Functioning
We examined the association of each indicator of prestroke functioning with poststroke depression. Patients with depression reported having worse sexual functioning (χ
2=9.21,
P=0.002) and greater disturbance in appetite (χ
2=13.15,
P<0.001), sleep (χ
2=18.35,
P<0.001), and energy level (χ
2=30.99,
P<0.001) before stroke than nondepressed stroke patients. Furthermore, patients with poststroke depression were significantly less involved in exercise prior to stroke than nondepressed stroke patients (χ
2=19.45,
P<0.001). Significant independent effects were found for prestroke exercise and sexual behavior on the functioning of stroke subjects (
Table 2). Because depressed groups did not vary significantly from nondepressed in prestroke domestic (χ
2=0.14, df=3,
P=0.99) and leisure time activities (χ
2=3.90, df=3,
P=0.27), self care functioning (dressing: χ
2=1.13, df=1,
P=0.29; bladder/bowel function: χ
2=0.28, df=1,
P=0.60), group social activities (χ
2=0.29, df=3,
P=0.96), and living alone status (χ
2=0.27, df=1,
P=0.6), the independent effects of these variables on functioning were not examined.
Characteristics of Noncompleters of the CES-D
Compared with stroke subjects who completed the CES-D, noncompleters of the CES-D did not differ significantly in gender (χ2=0.363, df=1, P=0.547) or race (χ2=1.78, df=1, P=0.182), but noncompleters were more likely to be older (t=3.94, df=1333, P<0.001) and more impaired, as indicated by significantly higher scores for motor weakness (t=17.58, df=1330, P<0.001), sensory impairment (t=17.28, df=1324, P<0.001), total neurological symptoms (t=11.60, df=1330, P<0.001), and lesion volume (t=8.0484, df=793, P<0.001). Further, a higher proportion of noncompleters were found to have dementia (χ2=47.77, df=1, P<0.001) and to have lower scores for exercise than the completers (χ2=29.17, df=3, P<0.001).
DISCUSSION
To our knowledge, this study is the first to make use of a large data bank resource to examine the relationship between depression and functional impairment in a relatively immediate poststroke period (7–10 days after stroke), controlling for the effects of neurological, medical, sociodemographic, and prestroke functioning variables on poststroke functional status. This cross-sectional evaluation of patients entered in the SDB study demonstrated that self-reported depression was associated with greater impairment in activities of daily living. Furthermore, this study found that the depression provided unique contributions to poststroke function. These findings are consistent with five previous studies that found greater functional impairment in depressed patients compared with nondepressed patients following stroke.
3–5,12,13 Interestingly, of these five studies, three
3,12,13 used the BI as a measure of functional ability, and three
3,5,13 assessed patients within 3 weeks of the stroke. This raises the issue of whether the type of functional measure used and/or the timing of the assessment after stroke influences the strength of the relationship between depression and functional impairment. Barthel measures were closely related to motor loss
27 and physical functioning,
33 so the observed significant effect of depression in patients immediately following stroke might involve largely the physical domain of functioning. We furthermore found no differences in functional impairment between severe and moderate depression, in contrast to a previous controlled study that demonstrated significant differences in functional abilities between major and minor depression.
34 There are several possible explanations for the discrepancies in these findings. First, the diagnosis of depression in the other study was made by using a structured psychiatric interview (modified PSE) and diagnostic criteria (DSM-III), while in this study the depression was determined by a self-rating instrument (CES-D). Second, we do not know whether the severity of depression in depressed stroke patients, as defined by self-reported CES-D scale, would be predictive of functional level. Third, because the BI focuses on physical functioning, the lack of a relationship between functional impairment and severity of depression in these depressed stroke patients suggests that physical dysfunction may not be a good indicator of severe depression. Perhaps psychosocial or global dysfunction might better distinguish between severe depression and moderate depression.
How can we account for the association of depression and functional impairment? Although these cross-sectional data do not allow us to examine the cause–effect relationship between functional impairment and poststroke depression, the observed lack of correlation between any particular group of depressive symptoms and functional impairment suggests that functional disability is not an epiphenomenon of psychomotor retardation in acute stroke subjects but instead is related to the whole syndrome of depressive illness. Although these findings cannot refute the argument that ADL impairment may lead to depression, the facts that lesion characteristics (laterality, volume) did not differ significantly between depressed and nondepressed subjects and lesion volume was not significantly correlated with BI scores suggest that large strokes with accompanying impairments were not the full explanation for depression. On the other hand, apathy or demoralization secondary to depression may have led to greater apparent functional deficit. Further, our data indicate that 52% of depressed stroke patients were functionally independent (BI>60) and 29% of nondepressed stroke patients were functionally dependent (BI<59), implying that severe impairments do not always lead to depression and relatively well-functioning stroke patients may suffer from depression. This would suggest that depression is not simply a reaction to the severity of functional impairment.
An important finding of this study is that weakness scores were shown to have independent negative effects on the functioning of all stroke patients, supporting the assumption that motor loss is a significant contributor to functional impairment in stroke patients. Further, the demonstrated significant association of poor prestroke physical functioning with poststroke depression as well as functional impairment merits further discussion. Depression may have been present in many of these patients before their stroke, or prestroke poor physical functioning might be related to the severity of medical conditions or preexisting personality attributes. Further, subjects with poor physical functioning may be prone to develop stroke and depression, and it is also conceivable that prestroke functioning influences the poststroke functional consequences. It is worth noting that age emerged as an independent factor that negatively affected the functional abilities of stroke patients. This is consistent with previous findings that older age is associated with decrements in functioning in poststroke patients.
35We recognize that the findings from the SDB are limited by several factors. First, the SDB involved hospitalized patients with acute stroke, and it therefore was not a random sample of all stroke patients. Second, less than half of the SDB patients completed the CES-D, and noncompleters were more impaired and older than completers. Thus, our sample is not representative of the entire stroke population, limiting the validity of inferences beyond the sample analyzed. Third, there may have been site differences in the detection of depression. Whereas 35% of the stroke patients enrolled by the Baltimore center were found to be depressed, only 17% of stroke patients enrolled by the Boston center were found to be depressed. Fourth, the existence of depression was defined by a cutoff score on the CES-D and was not determined by means of a structured psychiatric interview and defined diagnostic criteria. This method may have led to overidentification of depression in some patients and a failure to recognize depression in others. Fifth, the BI, used in this data bank to evaluate functioning, is less sensitive to changes in functional independence during the acute phase than are global outcome scales that accommodate language and cognitive deficits.
36 Use of the BI might have weakened the strength of the observed association between variables, including depression and ADL, in stroke patients. With these caveats, however, this study represents the first attempt to conduct a multicenter study of poststroke depression.
In summary, the results of this study suggest that of all the factors, depression may be the only treatable condition that independently affects the functioning of patients following acute stroke. The important clinical implication of the study is that depression should be taken into account in the evaluation and treatment of functional abilities of all stroke patients. Further, our findings confirm previous observations that depression often goes undetected and untreated in stroke patients.
12 Several studies have suggested, and one open-label treatment trial has demonstrated, that poststroke depressed patients may have greater improvement in ADL if they are treated with antidepressants.
6,7,37,38 Hence, early recognition and effective treatment of depression following stroke might also optimize rehabilitation potential and thereby reduce the very significant human and financial costs associated with poststroke functional impairment. Further research is needed to determine how depression contributes to impairments in ADL and which impairments respond to antidepressants.
ACKNOWLEDGMENTS
This study was supported in part by a grant from the Canadian Psychiatric Research Foundation (R.R.), NIMH Research Scientific Award MH00163 (R.G.R.), and Grants No1-2-NS-2398 for the Stroke Data Bank. The authors thank Mary Knickle for technical assistance.