Rapid-rate transcranial magnetic stimulation (rTMS) allows for the noninvasive examination of the cerebral cortex.
1 rTMS stimulates the cerebral cortex with magnetic fields resulting from current passing through a wire coil on the scalp. Transcranial magnetic stimulation has been used to investigate brain functions such as attention, memory, movement, speech, and vision. In addition, rTMS is beginning to be used to investigate the pathophysiology of Parkinson's disease.
1On the basis of these results, along with observations in functional and structural imaging studies that frontal lobe abnormalities exist in some patients with depression, rTMS studies have begun to investigate whether rTMS may be therapeutic for the treatment of depression.
4 The initial reports using TMS for the treatment of depression were “open label” and stimulated the center of the head. The results of these early studies were promising.
5–7 Subsequently, George et al.
8 at the National Institute of Mental Health employed rTMS over the prefrontal cortex in 6 treatment-refractory depressed patients and observed improvement in 2 of their patients. The largest rTMS study of depression to date was done by Pascual-Leone et al.
9 They used a crossover placebo-controlled study design. The authors reported that left prefrontal rTMS was effective in treating 11 of 17 young (less than 60 years of age) depressed patients. All of the patients were either medication resistant or psychotic. Interestingly, left prefrontal rTMS was found to be more effective than rTMS using right-sided or more posterior anatomic stimulation sites. None of the patients in this study experienced any significant side effects from rTMS.
On the basis of these studies, we decided to conduct an open, prospective trial that examined the use of left prefrontal rTMS in the treatment of patients with refractory depression. The goals of this study were:
METHODS
Subjects
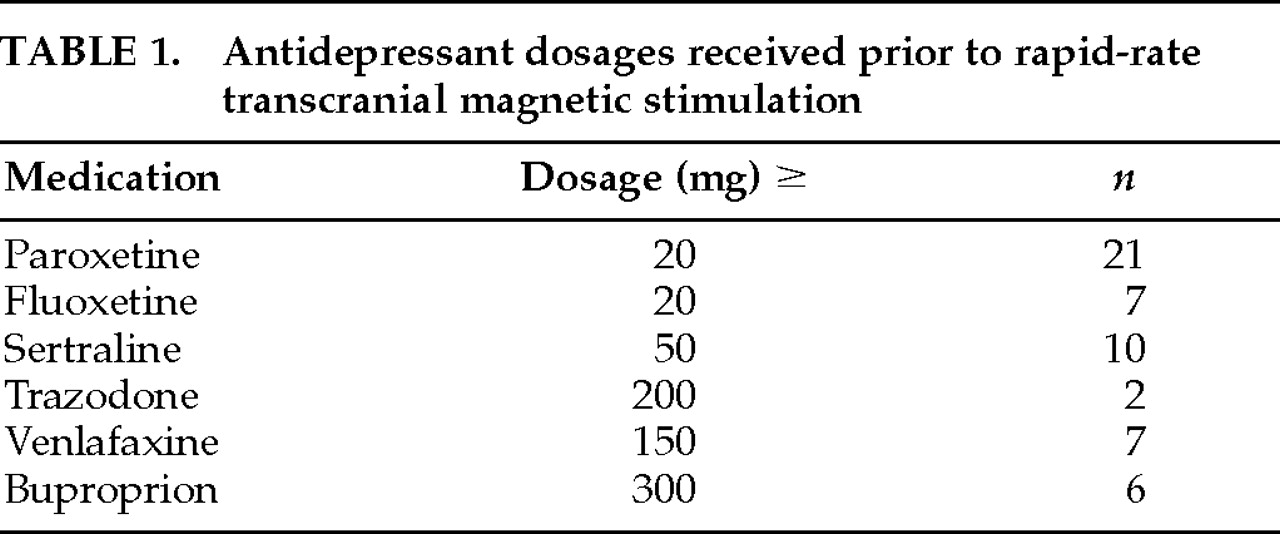
Between January 1996 and March 1997, 56 patients received a course of rTMS at Wesley Woods Geriatric Hospital/Emory University Medical Center. All 56 patients and their families gave informed consent to a protocol approved by the Human Investigations Committee of the Emory University School of Medicine and by the research committees of both hospitals. All 56 patients were referred to the Mood Disorders Clinic at Wesley Woods Geriatric Hospital. Forty-four of these patients had been referred for electroconvulsive therapy (ECT) and chose to receive rTMS prior to ECT. Fifty-three of the patients (94%) had received at least a 6-week trial of an antidepressant at a therapeutic dose (
Table 1). Seventeen patients had a prior history of receiving ECT. There were 31 women and 25 men among the 56 patients who received rTMS. The patients ranged in age from 22 to 89, with a mean age of 60 years.
All patients met DSM-IV criteria for a major depressive episode (53 unipolar, 3 bipolar). All patients were rated at least moderately ill on the Clinical Global Impression Scale and had a pretreatment score on the 21-item Hamilton Rating Scale for Depression (Ham-D) of greater than 20 (mean score of 34). In addition, 4 patients had a DSM-IV diagnosis of dementia. All diagnoses were made by a physician (G.S.F.) using a DSM-IV checklist during a structured clinical interview. The Folstein Mini-Mental State Examination (MMSE) was administered to 54 of the 56 patients before they received rTMS administered by a member of the treatment team (J.A.-L.). Two patients were not able to perform the mental status examination because of the severity of their illness.
In general, patients were tapered off their psychotropic medications prior to beginning their course of rTMS. A few patients (n=6) were unable to be tapered off their medications because of the severity of their illness. In no case was a patient started on a new psychotropic medication during rTMS treatments. The patients who remained on psychotropic medications had been on these medications for several months. As a result, any change in these patients' clinical status during rTMS was not felt to be attributable to the concomitant use of the psychotropic medications.
Many (n=21) of the patients had a history of one or more of the following medical illnesses: hypertension and/or coronary artery disease (n=21), congestive heart failure (n=2), history of transient ischemic attacks (n=3), diabetes mellitus (n=6), or cerebral vascular accident (n=1). Patients with a history of a recent myocardial infarction, cardiac pacemaker, intracranial metallic objects, or an increased intracranial pressure were excluded from the study.
The patients were classified as responders according to the criteria of Sackeim et al.
11 They had to show a 60% reduction from their pretreatment Ham-D score, as well as a posttreatment Ham-D score of 16 points or less. In addition, patients had to be rated as moderately to markedly improved on a 7-point Clinical Global Impression Scale. These ratings were completed by the patient's clinical treatment team along with the physician on the rTMS service (G.S.F.). All ratings were obtained prior to beginning rTMS and within 48 hours after the fifth treatment.
rTMS Methods
All treatments were administered in the ECT laboratory, once daily for 5 consecutive weekdays. If there was no clinical response after 5 treatments, rTMS was stopped. Patients lay supine with the head elevated on a pillow. Continuous cardiac monitoring was performed, and blood pressure was taken every 60 seconds during stimulation.
High-speed rTMS was performed with a custom device, using a damped cosine pulse and a prototype iron-core magnetic coil. The latter induces electric fields similar in distribution to those from a Cadwell water-cooled figure-eight coil but is much smaller, quieter, and more efficient, requiring no special cooling. This coil also has an open port in the center for precisely marking and reproducing position on the head.
Relaxed motor threshold and the location of the right-hand motor area were identified over the left hemisphere as previously described.
12 During stimulation at a rate of 1.0 Hz, the magnetic coil was moved across the left central region and the stimulator output was gradually increased to locate the point of lowest-intensity activation, followed by the magnetic threshold at that site. This position was then labeled with a permanent marker. Determining motor threshold required only 5 to 10 minutes at the first treatment session, and less time on subsequent days because the location had been marked. The site of rTMS treatment was then measured 5 cm anteriorly from the hand motor area on a sagittal line.
For each rTMS treatment, the system output was set to 110% of relaxed motor threshold and a repetition rate of 10 Hz. Stimulation was delivered in ten trains of 5 seconds each, with trains 30 seconds apart. The coil was oriented so that electric fields were induced to a sagittal plane. Ear protection was worn throughout.
Statistical Tests
Data were stored on a Lotus program. Comparisons on parametric and nonparametric variables were made by using Student's t-tests and chi-square tests, respectively.
DISCUSSION
The main finding of this study is that rTMS may be used safely and effectively in some patients with refractory depression. However, with the open-label design of this study, a placebo effect cannot be ruled out in the patients who responded to rTMS. In our study, 21 of 50 patients with depression (42%) responded to rTMS. In the largest rTMS study of depression to date, Pascual-Leone et al.
9 reported that 11 of 17 depressed patients (65%) responded to rTMS. Consistent with the Pascual-Leone study, the majority of patients in our study were medication resistant. In fact, all but 3 of our patients had failed a 6-week trial of an antidepressant prior to receiving rTMS (
Table 1).
Recently, Prudic et al.
13 have reported that ECT may be less effective in patients who have not responded to a therapeutic trial of antidepressants pre-ECT. Given that nearly all of our patients had received a therapeutic trial of an antidepressant pre-rTMS, we were unable to examine the response rate from rTMS in a less refractory group of depressed patients. Clearly this is an area in need of further research.
The rTMS protocol used in our study was similar to that of Pascual-Leone et al.
9 In both studies the therapeutic benefits of rTMS were observed after 5 rTMS treatments by stimulating the left prefrontal cortex. In addition, both studies used a frequency of 10 Hz. We used a higher magnetic field intensity (110% motor threshold) than was used in the Pascual-Leone study (90% motor threshold) and a shorter stimulus train with far fewer total pulses per session (500 vs. 2,000 pulses, 5 vs. 20 minutes). Clearly, additional placebo-controlled rTMS depression studies are required that examine the potential effects of different stimulus intensities, train durations, stimulus frequencies, number of treatment sessions, and anatomic stimulation sites on the therapeutic response from rTMS.
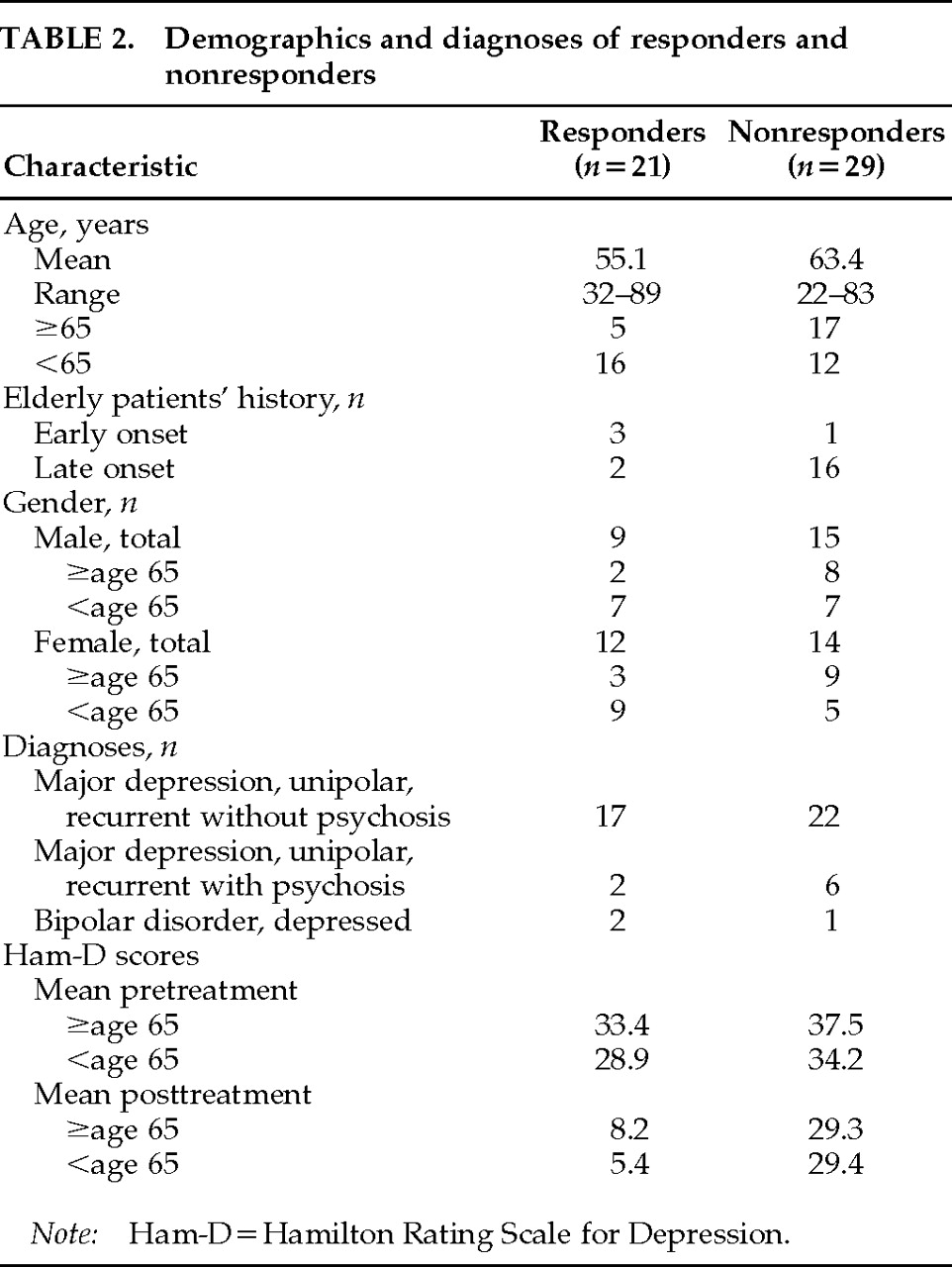
All of Pascual-Leone and colleagues' patients were under age 60, whereas in our study 22 of the subjects were 65 years of age or older. Interestingly, 16 of the younger patients (56%) responded to rTMS, whereas only 5 of the 22 elderly, depressed patients (23%) responded to rTMS. Furthermore, among the depressed elderly patients, 3 of the 4 (75%) who had their first episode of depression prior to age 65 (early onset) responded to rTMS, but only 2 of the 18 (11%) with late-onset depression (first episode after age 65) responded to rTMS. We have previously reported that there is an increased incidence of structural brain changes in elderly depressed patients and that elderly patients with late-onset depression have the most severe structural brain changes.
14,15 These findings may explain the poor response from rTMS observed in our elderly depressed patients. At our institution, we are currently examining whether preexisting structural brain changes in the depressed elderly may predict a poor response from rTMS.
Consistent with Pascual-Leone's study, we did not find that a positive response to rTMS was related to the younger patients' Ham-D scores. In addition, there was no difference in gender or pre-rTMS MMSE scores between responders and nonresponders in this study. Sixteen patients reported a favorable response to ECT in the past. Of these, only 8 responded to rTMS. At our institution, we are currently examining whether nonresponders to rTMS are less likely to achieve a therapeutic response from ECT.
Although the antidepressant mechanisms of action for ECT and rTMS remain unknown, recent works are beginning to examine the neurochemical basis for rTMS and its effects on several animal behavioral models.
16–18 Both ECT and rTMS have been shown to reduce immobility in the forced swimming test. In addition, enhanced apomorphine-induced stereotypy was increased by both rTMS and ECT, implying a dopaminergic effect in both treatments. While rTMS is a nonconvulsive method for inducing depolarization of neurons in the brain, TMS has also been shown to inhibit seizure activity, decrease seizure duration, and increase the seizure threshold for additional ECT-induced seizures in animals; these findings are not unlike the anticonvulsant properties observed with ECT. Similar to ECT and antidepressants, rTMS may alter brain monoamines. Regional alterations in dopamine, serotonin, and 5-hydroxyindoleacetic acid levels have been reported with rTMS.
Finally, like antidepressants and ECT, rTMS can significantly decrease the number of beta-adrenergic receptors in certain parts of the rat's brain.
18 On the basis of these works, it is not unreasonable to expect that further rTMS studies will enhance our understanding of the pathophysiology of depression and may ultimately lead to the development of safer, more effective treatments for depression.
In both Pascual-Leone's report and ours, no patient reported a new onset of seizures during rTMS treatments. However, in 2 patients during rTMS, muscular contractions spread from a single hand muscle to more proximal muscles in the right upper extremity. This may have represented intracortical spread of excitation. This spread of excitation in the primary motor cortex could be an indicator of the potential epileptogenicity of rTMS in individual patients.
1 Neither of these individuals developed any neurologic sequelae. This spread of muscular excitation has been associated with higher frequencies of stimulation (≥5 Hz) during rTMS. Future rTMS research will be needed to examine the risks and benefits of high-frequency versus low-frequency rTMS treatments for depression. Headaches were the most common complaint; however, these were minor in most patients. Only 2 patients asked to discontinue rTMS because of the pain elicited during rTMS stimulation. There were no observed cardiovascular complications in any patients. This is of potential significance given the high incidence of preexisting coronary artery disease in many of our elderly patients.
Finally, none of our patients complained of memory or cognitive side effects from rTMS. These observations are consistent with previous safety reports on rTMS.
19–21 However, additional neuropsychological studies of rTMS are indicated to further examine this area.
In summary, rTMS appears to be safe and effective in treating some medication-resistant depressed patients. However, the therapeutic benefits of rTMS appear to be greater in younger patients. Future work is needed to examine ways to sustain the therapeutic benefits of rTMS and to identify the optimum techniques for its administration. The potential radiological and clinical predictors of response to rTMS will also need further study.