The neuropsychiatric disturbance in patients with Alzheimer's disease (AD) is a major cause of anxiety and concern for caregivers and a frequent cause of institutionalization. Appropriate management of behavioral symptoms may lessen the burden of caregivers and may postpone institutionalization. Therefore, evaluation and management of behavioral symptoms are of considerable importance in practice.
Delusions are, in particular, a concern for management of AD. DSM-IV
1 has classified dementia of the Alzheimer's type into subtypes with or without delusions. In a review of 21 studies, Wragg and Jeste
2 reported that frequencies of AD patients who had experienced delusions at some time in the course of their illness ranged from 10% to 73%; the greatest number of studies had clusters between 30% and 38% (median=33.5%). The underlying mechanism for delusion formation in AD is still unclear. Although delusions in AD may be an “understandable” reaction to intellectual deficits,
3 evidence of involvement of a specific brain area is increasing in other neurologic disorders.
4 However, efforts to explore the relationship between delusions and focal brain dysfunction in AD patients using SPECT or PET are limited, and have demonstrated conflicting results; delusions or other psychiatric symptoms in AD or other dementias were associated with frontal hyperperfusion in combination with hypoperfusion in the posterior temporal region,
5 with hypoperfusion in temporal lobes,
6 with left frontal hypoperfusion relative to the opposite side,
7 with right anterior temporal hypoperfusion,
8 with bilateral parietotemporal
9 or frontal
10 glucose hypometabolism, or with hypometabolism in bilateral orbitofrontal and cingulate areas and left medial temporal areas and normalized hypermetabolism in bilateral superior temporal and inferior parietal areas.
11 These conflicts may be due to differences of experimental design, including selection criteria, sample size, and method for determination of cerebral blood flow or metabolism.
We postulated that if neuroanatomical substrates were underlying delusions in AD, distinctive features in regional cerebral metabolism that differentiate the patients with delusions from those without delusions would be found in the limbic and/or association cortices. To investigate this hypothesis, we prospectively examined 65 AD patients for delusions and explored the relationship of delusions in AD with regional cerebral glucose metabolism (CMRglc) as determined by PET.
RESULTS
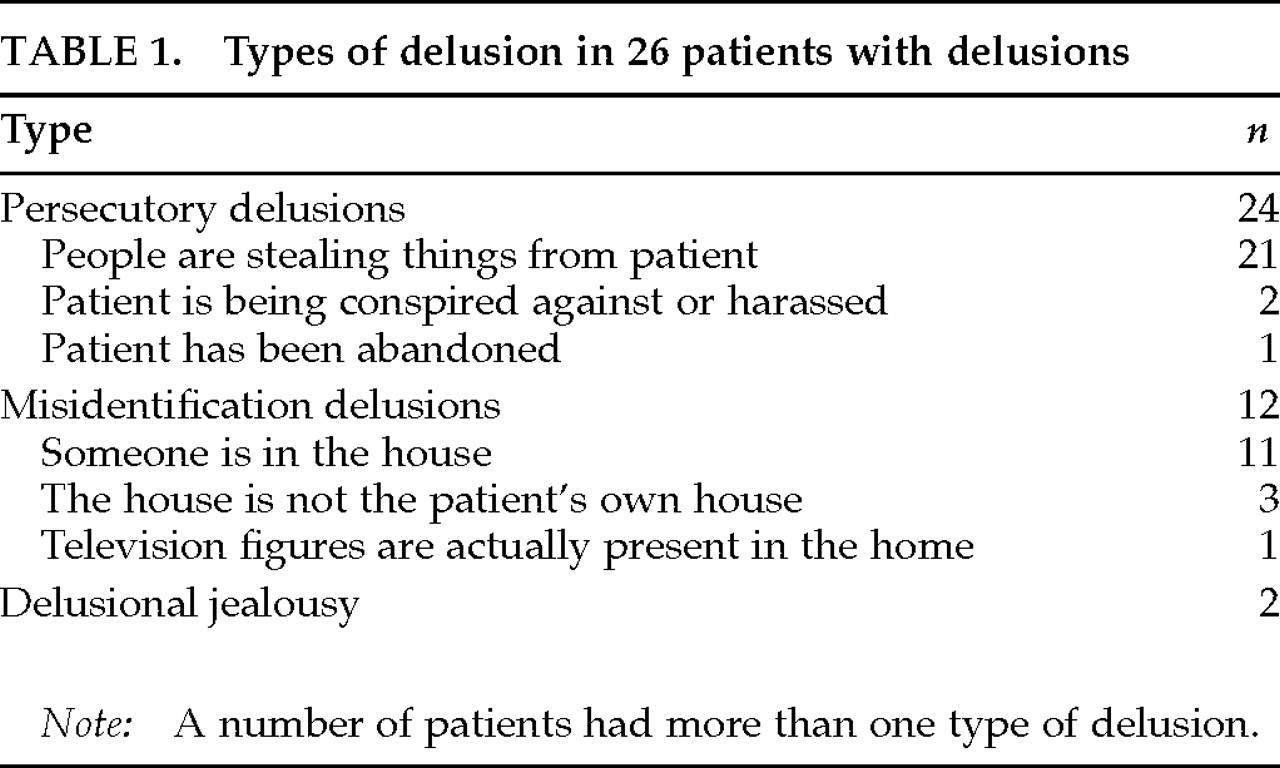
Delusions were present in 26 patients (40.0%). The types of delusions are summarized in
Table 1. Thirty-nine patients were free from delusions. Among patients with delusions, 7 had hallucinations (visual hallucinations in 6 patients, visual and auditory in 1); among those without delusions, 1 had visual hallucinations (Fisher's exact probability test,
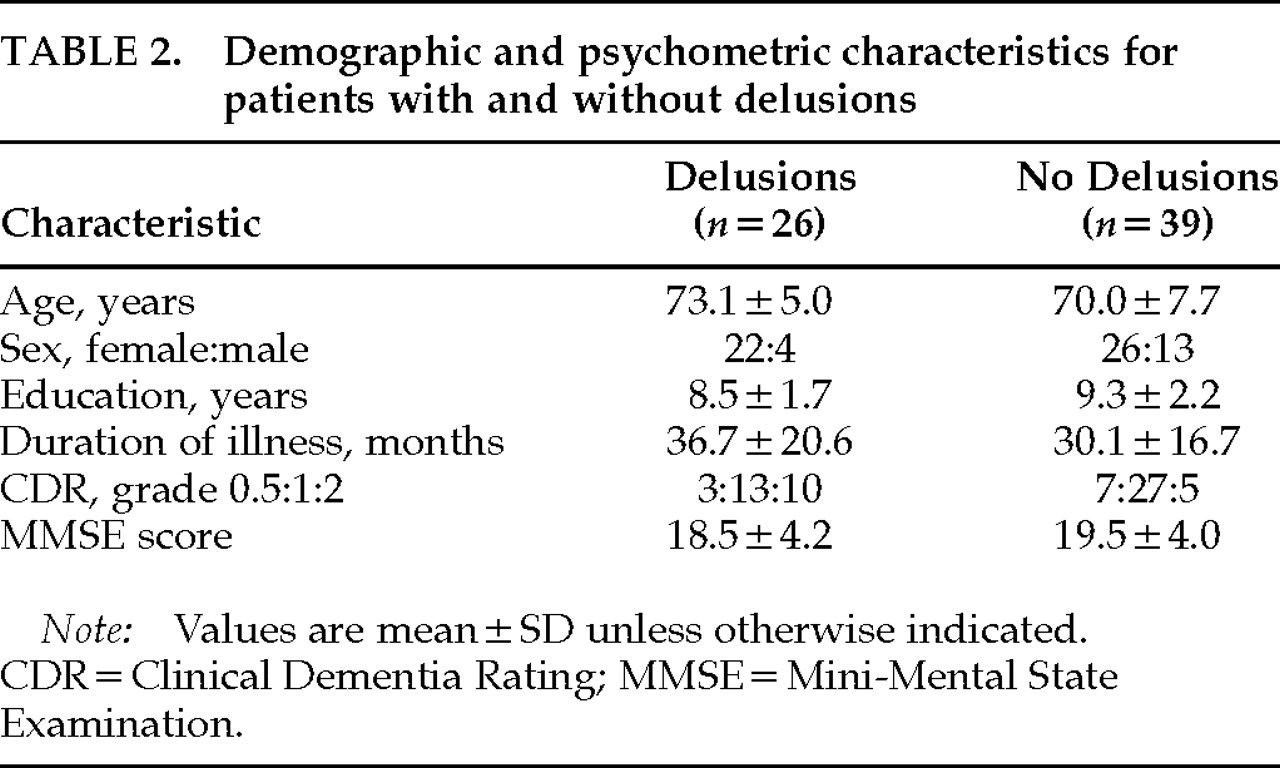
P=0.0054). However, experience of hallucinations was not reported during the PET examination. No significant differences were found between those with and without delusions in age, sex, years of education, duration of illness, or severity of dementia (
Table 2).
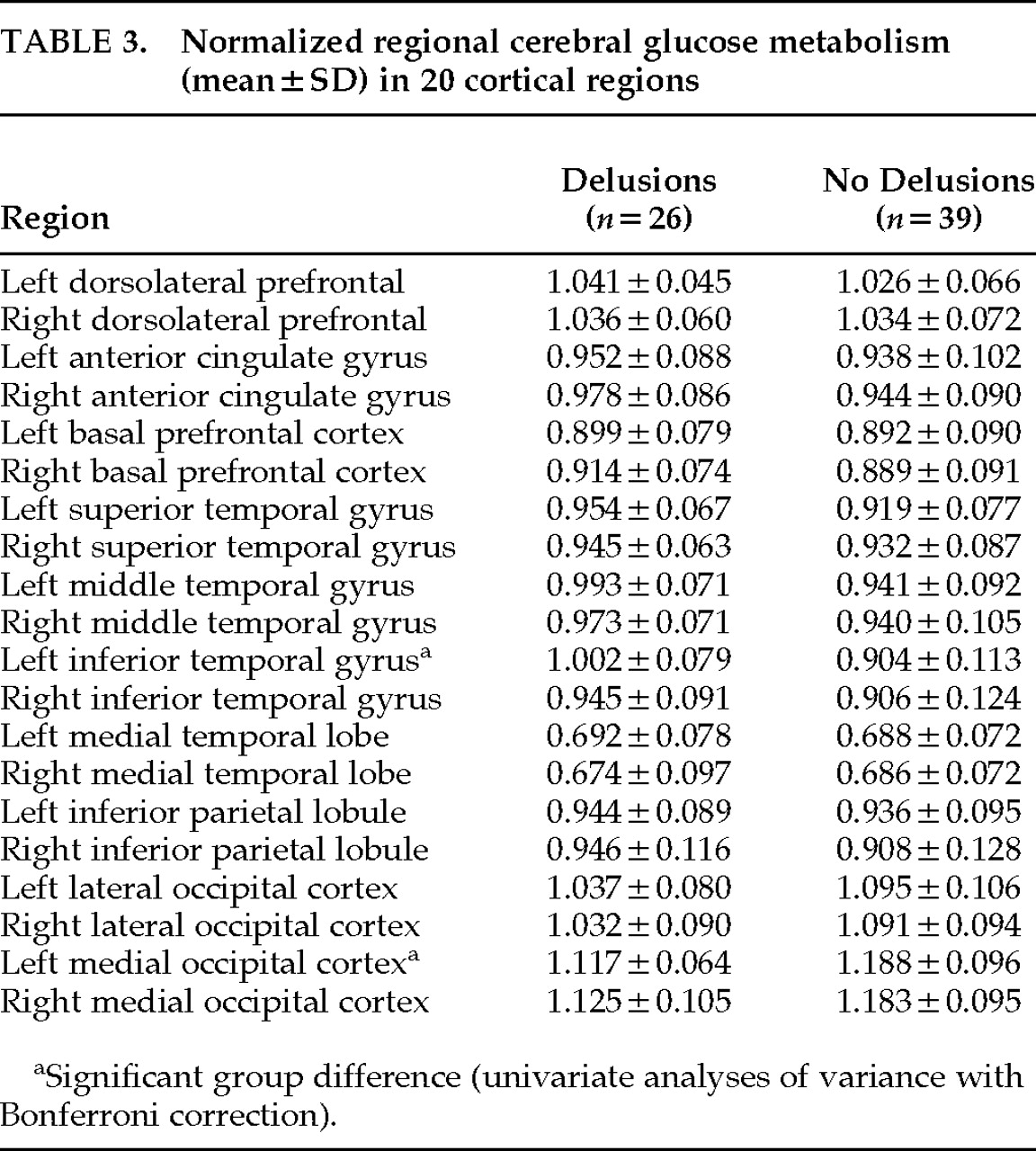
The means and standard deviations of nCMRglc in each region are summarized in
Table 3. The repeated ANOVA revealed no significant effect of group (patients with and without delusions) in global metabolic activity (
F=3.83, df=1,63,
P=0.055), but a significant effect of brain region (
F=108.4, df=19,1197,
P<0.0001). The group×region interaction was highly significant (
F=3.44, df=19,1197,
P<0.0001). After Greenhouse-Geisser adjustment, this interaction effect remained significant (
P=0.0019). Post hoc univariate ANOVAs with Bonferroni correction demonstrated that the left inferior temporal gyrus (
F=14.75, df=1,63,
P=0.0003) and the left medial occipital region (
F=10.83, df=1,63,
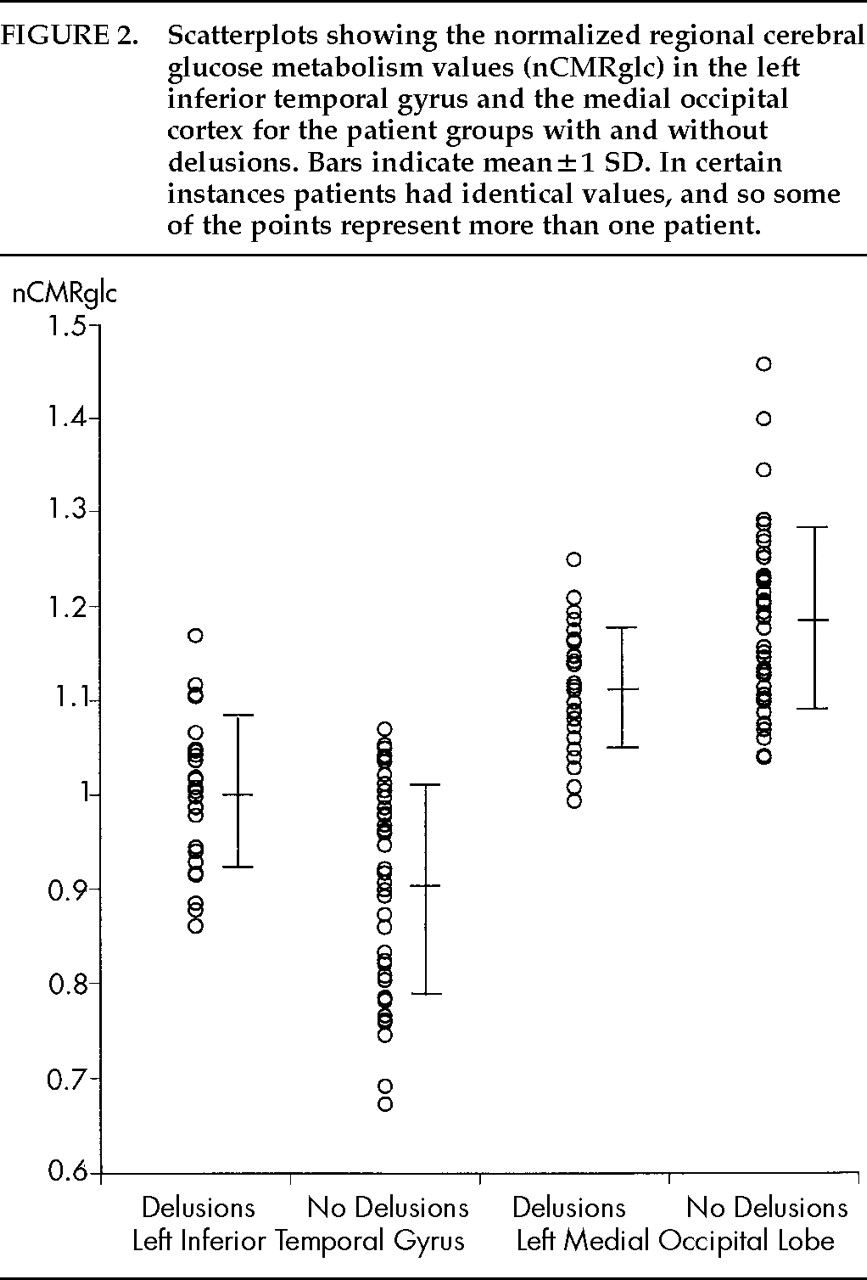
P=0.0016) significantly contributed to this interaction effect; the mean nCMRglc was significantly increased in the left inferior temporal gyrus and was significantly decreased in the left medial occipital region in those with delusions (
Table 3;
Figure 2). Data for patients with hallucinations did not make specific clusters in either region. There was a significant inverse correlation of nCMRGlc between the left inferior temporal gyrus and the left medial occipital region (Pearson
r=–0.464,
P<0.0001).
DISCUSSION
Several studies carried out to determine a focal abnormality associated with delusions in AD have reported conflicting results. Gustafson and Risberg,
5 examining presenile dementia not specific to AD by using intra-arterial
xenon-133 injection technique, found a high gray matter blood flow, especially in the frontal lobe, in combination with a decreased flow in the posterior temporal region, in patients with paranoid symptoms. Starkstein et al.,
6 using [
99mTc]HMPAO SPECT, demonstrated that the mean cerebral blood flow significantly decreased in the temporal lobes in AD patients with delusions compared with those without delusions. Using the same technique, Kotrla et al.
7 reported that AD patients with delusions had hypoperfusion in the left frontal lobe as compared with the opposite side. In their small-sized but longitudinal study, Pontón et al.
8 demonstrated rapid deterioration of cerebral blood flow in the right anterior temporal regions in AD patients with delusions compared with those without delusions. The main feature of patients' delusions in their study was Capgras syndrome, which is reportedly associated with lesions in right cerebral hemisphere.
21,22 The study by Grady et al.,
9 retrospectively analyzing 33 patients with AD who received PET scans, demonstrated that bilateral parietotemporal metabolic deficits were associated with psychotic symptoms. Sultzer et al.,
10 evaluating 21 patients with AD by using the Neurobehavioral Rating Scale and FDG PET, found that psychosis was significantly correlated with frontal glucose hypometabolism. Mentis et al.
11 reported that AD patients with delusional misidentification syndrome, when compared with those without this syndrome, had significant glucose hypometabolism in bilateral orbitofrontal and cingulate and left medial temporal areas and significant normalized hypermetabolism in the bilateral superior temporal and inferior parietal areas. Possible causes of the disagreement among those studies include different techniques for regional cerebral blood flow or metabolism measurement, different distribution of delusions or psychoses, different statistical techniques, and small-sized or retrospective analysis.
Several strengths of our study can be noted. First, conservative statistical analyses were employed for a relatively large (the largest) number of patients. Second, the variables that may influence regional CMRglc, including age, sex, handedness, and severity of the disease, were comparable between those with and those without delusions. Third, the type of delusions was unbiased (persecutory delusion is common in AD
23,24). Finally, a possible effect of hallucinations on PET results was unlikely because hallucination was absent during the PET examination; a relatively small proportion of patients, if any, were affected.
We found a modest but significant normalized glucose hypometabolism in the left medial occipital region and a modest but significant normalized glucose hypermetabolism in the inferior temporal gyrus in the patients with delusions. This finding supports the hypothesis that delusions in AD are associated with dysfunction in specific brain areas.
An association between psychotic symptoms and dysfunction in each region has been repeatedly demonstrated in other neuropsychiatric disorders. A medial occipital involvement often produces agitated delirium after stroke. Severe agitated delirium, where delusions are main features in addition to hallucinations and emotional and autonomic excitement,
25 occurs after unilateral or bilateral medial occipital lesions with or without posterior temporal lesions due to posterior cerebral artery territory ischemia.
26–29 In some studies, left-sided predominance of lesions has been stressed.
27,29 These findings on focal medial occipital destruction are consistent with our results, and they support the notion that dysfunction in this region, as well, is attributable to the development of delusions. Increased activity in the left temporal lobe related to positive psychiatric symptoms has been repeatedly pointed out in schizophrenic patients. DeLisi et al.
30 reported that patients with chronic schizophrenia had significantly greater CMRglc in the left than the right anterior temporal lobe and that the extent of this lateralization was in proportion to the severity of psychopathology. Liddle et al.,
31 examining psychiatric symptom scores and regional cerebral blood flow with PET in schizophrenic patients, found that the reality distortion factor scores that were loaded by delusion and hallucination scores correlated positively with regional cerebral blood flow in the left temporal region. Furthermore, Kaplan et al.,
32 in a study of untreated schizophrenic patients, reported correlation between the reality distortion factor scores and relative CMRglc in the left temporal region. Our results that relative hyperactivity in the left inferior temporal gyrus was accompanied by delusions suggest that dysfunction in the neuronal networks, as occurs in schizophrenia, is conceivable in AD.
Cummings
33 postulated, from data on anatomical sites of pathology across a variety of organic brain disorders, that delusions arise from the disruption of connections between limbic and cortical areas. Krieckhaus et al.
34 proposed that delusions of paranoid schizophrenia are attributable to a hyperactivity of the dopamine-sensitive hippocampal CA1 neurons, which generates a diffuse reinforcement signal via the inferior temporal cortex and thus is responsible for the fixation of belief in the parietal-temporal-occipital association cortices. Devinsky et al.
27 suggested that damages involving the posterior hippocampus and parahippocampal and occipitotemporal gyri destroy the specific connections between the neocortex and limbic system, causing delirium. These arguments are consistent with our results, suggesting that malfunction of the network system involving the hippocampus, the inferior temporal cortex, and the medial occipital cortex may account for delusions in AD. The negative correlation of metabolism between the inferior temporal cortex and the medial occipital cortex may support the functional interaction between these areas.
The magnitude of group difference was modest and the statistical analysis applied was very conservative, suggesting dysfunction in other brain regions might be involved in the development of delusions. If delusions had been present during PET scanning, the group difference might have been more marked. Despite these limitations, the findings of significant differences in normalized regional cerebral metabolism between the patients with delusions and those without delusions under such conditions support a hypothesis of association between delusions in AD and cortical dysfunction.