Normal pressure hydrocephalus (NPH) was first proposed in 1965 as a form of communicating hydrocephalus that could result in a treatable dementia.
1,2 Hydrocephalus refers to dilation of the ventricles of the brain. It often results from an obstruction to the normal flow of cerebrospinal fluid (CSF) causing CSF to accumulate in the ventricles (obstructive hydrocephalus). In communicating hydrocephalus, CSF flow is not blocked within the ventricles, but it accumulates because it is not absorbed properly. In NPH, the CSF pressure is generally within normal values. The prevalence of NPH in the general population has not been quantified. Two European studies in small populations have roughly estimated that 1% to 6% of all dementias are due to NPH and 0.41% of persons in the general population 65 years or older have the disease.
3,4 However, both groups felt that NPH is significantly underestimated because many cases go unreported and untreated.
NPH has a classic symptom triad of gait disturbance, urinary incontinence, and neuropsychological impairments that are most often labeled as dementia. This dementia of NPH has not been standardized with specific criteria. However, common symptoms include severe somnolence, fatigue, emotional lability, cognitive dulling (mild to severe), and memory impairments. These changes in memory can be mild or as severe as those of Korsakoff's syndrome. In fact, some authors define the memory disorder of NPH as a Korsakoff's amnesia (anterograde and retrograde memory deficits with confabulations).
5,6 Other disabilities include episodic mutism, hypokinesia, or catatonia. More rarely, cases of ultra-rapid mood cycling, disinhibition, aggression, delusions, hallucinations, and depression have been reported.
7,8The gait disturbance of NPH has been traditionally classified as “apraxia,” or cortical inability to direct movement. Recent computerized analyses of gait patterns have led some researchers to propose that the slowness of step, decreased step height, truncal flexion, and decreased pelvic rotation of NPH are more similar to Parkinson's disease than to a cortical motor disorder.
9 These descriptions and other case reports describing bradykinesia, rigidity, dystonias, and tremors have led to further examination of the pathogenesis of NPH.
10Although most cases of NPH are idiopathic, it can be associated with many conditions, including trauma, subarachnoid hemorrhage, prior intracranial surgery, and meningitis. There are two primary theories for the origin of NPH. The first is that it is an obstructive yet communicating type of hydrocephalus with a blockage of CSF resorption.
11 The other is that it results from weakening of the ventricular wall subsequent to periventricular white matter ischemic damage.
12 Whatever the precipitating events, as the ventricles begin to enlarge, the surrounding white matter is pressed outward. The fibers of the coronal radiata are damaged by the tangential shearing forces generated by the expansion and contraction of the brain during the cardiac cycle. In addition, cerebral blood flow may be impaired as vessels are stretched and compressed, resulting in ischemic damage. With continued ventricular expansion, the cortex is compressed against the inner table of the skull.
1,11,12 With prolonged compression comes further cortical and subcortical damage (e.g., nigrostriatal axis impairment) and the appearance of symptoms.
10 Positron emission tomography (PET), xenon-enhanced computed tomography (xenon-CT), and single-photon emission computed tomography (SPECT) studies all indicate widespread cortical and subcortical hypometabolism and impaired cerebral blood flow.
13–15Diagnosis of NPH can be made based on the presence of all or a portion of the clinical triad (gait disturbance, dementia, urinary incontinence) on examination, combined with lumbar puncture and diagnostic imaging findings. In NPH, lumbar puncture reveals a mean CSF opening pressure within the range of normal variation (<180 mm H
2O or 13 mm Hg with the patient in the lateral decubitus position). If resistance to CSF outflow is tested with an infusion of isotonic Ringer-lactate solution, the outflow resistance will be increased.
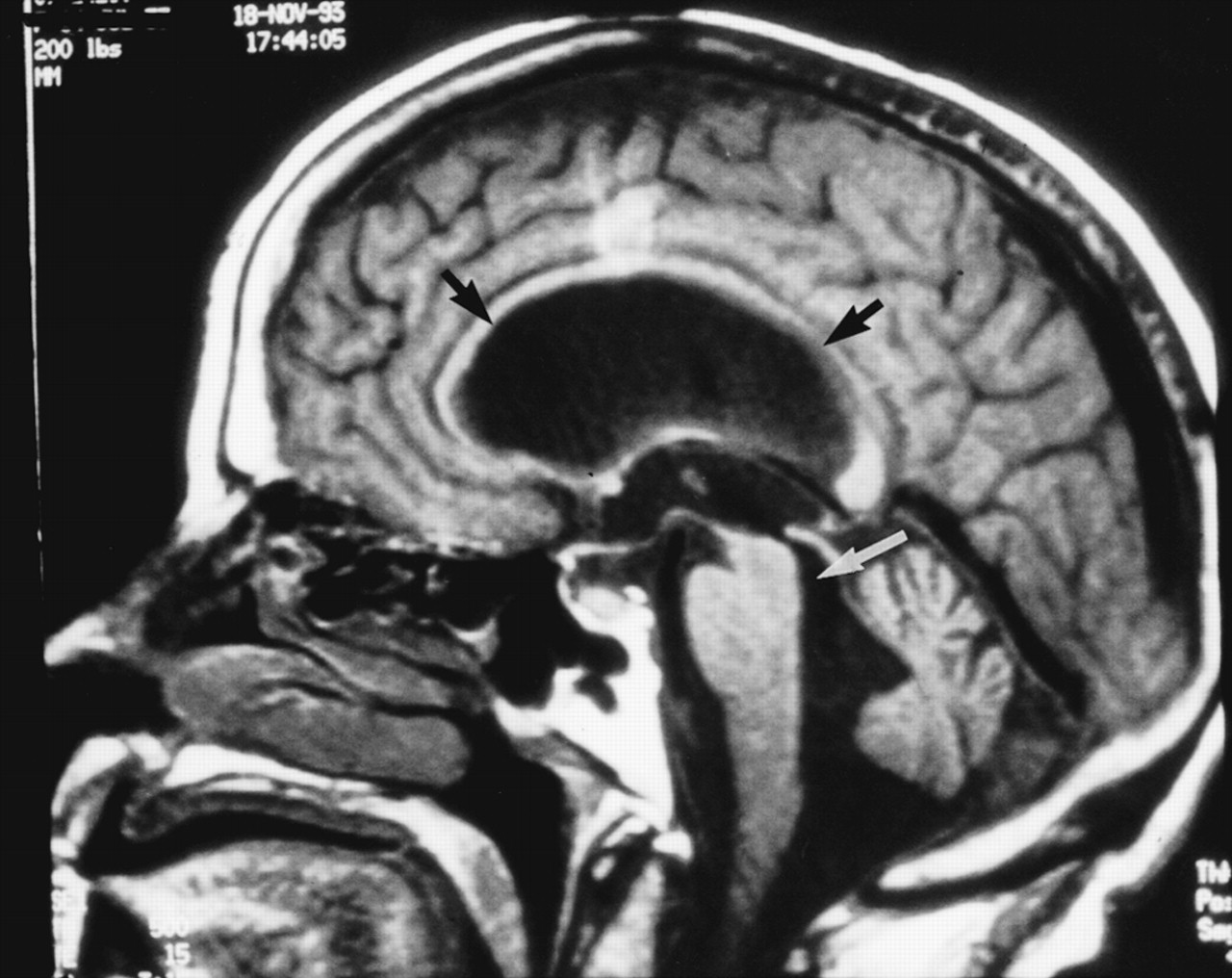
16,17 Diagnostic imaging reveals ventricular enlargement without sulcal widening. Ventricular enlargement is generally most evident in the frontal and temporal horns of the lateral ventricles. The corpus callosum may be bowed upward and the cerebral gyri flattened against the inner table of the skull. Periventricular white matter lesions are often present. Magnetic resonance imaging (MRI) is preferred over computed tomography because of its higher resolution, better delineation of pathology, and sensitivity to CSF flow dynamics.
The direction of CSF flow through the cerebral aqueduct reverses during the cardiac cycle. Thus, it is caudal (into the fourth ventricle) during systole and rostral (into the third ventricle) during diastole. MRI is very sensitive to fluid motion. This normal movement of CSF results in a loss of signal (flow void) within the cerebral aqueduct. CSF flow in NPH is hyperdynamic, with an increase in the amount and velocity of CSF passing rostrally, then caudally, through the cerebral aqueduct with each cardiac cycle. This fluid movement is visualized on proton density–weighted MRI as an increased flow void in the cerebral aqueduct (that is, a flow void extending outside the aqueduct into the posterior part of the third ventricle and the anterior portion of the fourth ventricle).
18 When present, a hyperdynamic CSF flow void is a specific indicator of treatment responsiveness in NPH. However,
absence of an increased flow void is not a reliable indicator that NPH is
not present, since the appearance of the flow void is profoundly decreased by many of the commonly used MRI acquisition parameters (i.e., flow compensation and fast spin-echo techniques).
Therapeutic options for NPH are limited to surgical shunt placement, which dampens the pressure in the ventricular system during systole (peak pressure times). An indwelling catheter that includes a low- or medium-pressure one-way valve is inserted into one of the lateral ventricles. It commonly drains into the peritoneal cavity or the superior vena cava. Success rates range from 20% to 80%, with reports of symptom relief lasting up to 4 years.
5,19 However, postsurgical complications have been reported in up to 40% of patients.
19 Thus, identifying those patients most likely to benefit from shunting is of key importance. The most promising factors under investigation to predict shunt responsiveness include duration of presurgical symptoms (less than 6 months appears favorable) and onset of gait disturbance before dementia; temporary pre-shunt symptom relief from a CSF tap test (removal of 40 ml of CSF via lumbar puncture); and absence of significant cerebral vascular disease.
16,17,19 Cerebral blood flow greater than 20 ml per 100 g per min on xenon-CT, with impaired vascular response to acetazolamide in perivascular white matter only, has also been reported to indicate good response.
14 However, these results have not been replicated.
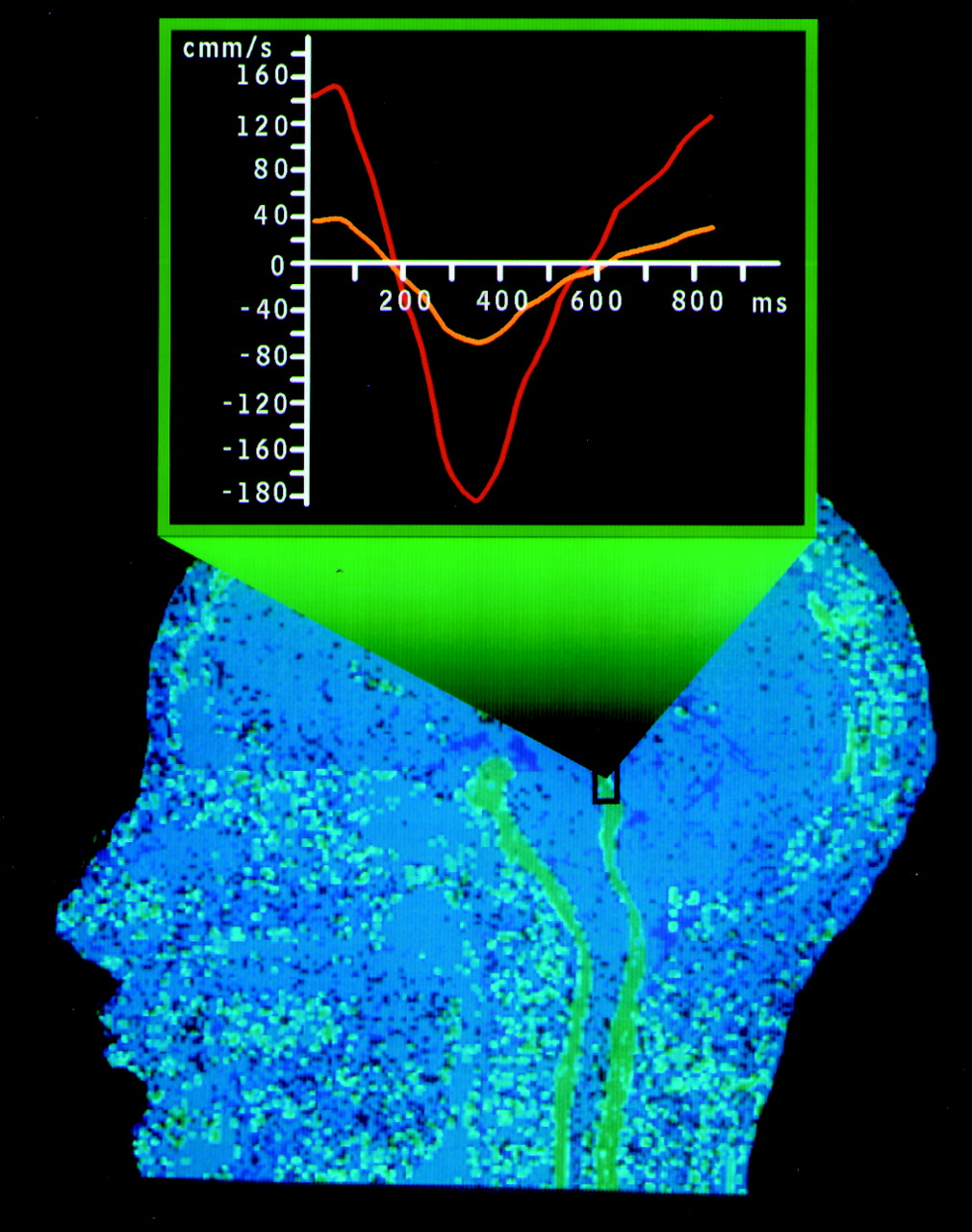
The recent development of phase-contrast CSF velocity imaging may provide a reliable method for identifying those patients with NPH who can be treated successfully. This method is a new MRI technique that allows quantification of CSF flow. Measurements are performed in the cerebral aqueduct, the narrowest portion of the ventricular system and therefore the location of highest CSF flow velocity.
20,21 Since CSF motion is pulsatile, the average of the volume of CSF moving caudally during systole and rostrally during diastole is calculated. This is defined as the CSF stroke volume. In the initial study with this technique, CSF stroke volumes above 42 μl were associated with a favorable response to shunting.
21 This is a promising tool for screening patients with clinical symptoms of NPH and thus identifying a treatable dementia without risking postsurgical complications in patients who will not benefit.