Postoperative delirium affects about 20% of the patients undergoing cardiac surgery, the estimates ranging from 3% to 47%.
1,2 It has been associated with several peri-operative risk factors, though not with any one factor consistently.
3–6Abnormalities of various neurotransmitter systems as a consequence of decreased oxidative metabolism in the brain have been implicated in the pathophysiology of delirium. According to this neurotransmitter hypothesis, delirium may be the result of reduced cholinergic function, excess release of dopamine, norepinephrine, and glutamate, and both decreased and increased serotonergic and gamma-aminobutyric acid activity.
7,8Changes in the levels of various amino acids that are precursors of cerebral neurotransmitters may affect their function and thus lead to delirium. For example, the production rate of brain serotonin (5-hydroxytryptamine; 5-HT) is dependent on the plasma availability of its precursor tryptophan (Trp); Trp competes with the other large neutral amino acids (oLNAA), such as tyrosine (Tyr), phenylalanine (Phe), valine (Val), leucine (Leu), and isoleucine (Ile), for transport across the blood–brain barrier (BBB). The ratio of Trp to the oLNAA eventually determines the amount of Trp that reaches the brain and consequently the synthesis of cerebral 5-HT.
9 Thus, delirium after cardiac surgery has been associated with reduced plasma tryptophan and, consequently, decreased cerebral 5-HT function.
10,11 Furthermore, phenylalanine is a precursor of dopamine and norepinephrine through a Tyr connection. The ratios of Tyr and Phe to the oLNAA determine the amount of Tyr and Phe that enter the brain. An increase in the cerebral uptake of Tyr and Phe may be a risk factor for the development of delirium if excess release of dopamine and norepinephrine is implicated in its pathophysiology.
Surgical trauma induces physical stress, increased activity of the limbic-hypothalamic-pituitary-adrenal axis, and a low triiodothyronine (T
3) syndrome. The latter is characterized by a decreased level of active T
3 as well as an increased level of inactive reverse T
3, in the absence of thyroid illness.
12 The degree of thyroid hormone alterations is dependent on the severity of disease or trauma, and it is both a measure of physical condition and a predictor of prognosis.
13 A low-T
3 syndrome very likely causes a generally decreased metabolism through reduced synthesis of adenosine triphosphate (ATP). This is another mechanism by which the production of 5-HT in the brain may be endangered, since Trp hydroxylase needs tetrahydrobiopterin as a cofactor, and the latter may be reduced in the presence of decreased ATP. Thus, surgery itself may contribute to an imbalance of neurotransmitters.
Surgery also causes an increase in corticosteroid levels. Cortisol is an important stress hormone that has modulating effects on both the limbic system and the immune system
14 and that inhibits the release of thyroid-stimulating hormone (TSH). Thus, changes in cortisol may influence brain function, immune function, and thyroid function and, through interacting mechanisms, may provoke delirium.
15 Surgery may endanger the transport of Trp across the BBB, first because the plasma concentrations of the oLNAA may be increased via degradation of muscle proteins, and second because elevation of corticosteroid levels induces Trp pyrrolase in the liver, reducing plasma Trp availability for the brain.
16In this study, we examined the interrelationships between the plasma levels of amino acids, physical condition, and postoperative delirium in patients undergoing elective cardiac surgery. We anticipated that delirium would be related to abnormalities in plasma amino acids. We also expected delirious patients, compared with nondelirious control patients, to be in poorer physical condition.
METHODS
Patients
All patients older than 21 years of age who were admitted for elective cardiac surgery (coronary artery bypass graft [CABG], valve replacement [VR], both CABG and VR, or other heart operations [OHO] such as surgery for heart defects with or without CABG and/or VR) were eligible to participate. From May 1991 through November 1992, 345 patients consecutively admitted to the Thoraxcentre of the University Hospital Rotterdam, which is a large, approximately 1,000-bed, inner-city teaching hospital in the Netherlands, were asked to join the study. Fifteen patients (4%) refused to participate. Of the 330 consenting patients, another 34 patients (10%) disappeared from the study for the following reasons: 6 patients died during or shortly after surgery, 7 patients underwent an operation elsewhere, 4 patients refused the day before surgery after having given informed consent, in 8 patients there were problems with blood sampling, and 9 patients were lost for various other reasons.
The study sample consisted of 296 patients, of whom 192 were men (65%) and 104 were women (35%). The mean age was 63 years (range 26–83, SD=11). The 296 study patients did not differ with regard to age and gender from the 49 patients who refused and dropped out. Informed consent was obtained from all patients. The study was approved by the ethics committee of the University Hospital Rotterdam.
Behavioral Assessments
Preoperative assessments were done 1 to 4 weeks before surgery and covered somatic and psychiatric history, current medical status, sociodemographic characteristics, and use of medication. We also administered the following test instruments: the Mini-Mental State Examination (MMSE
17) to screen for cognitive dysfunction; the 30-item version of the General Health Questionnaire (GHQ-30
18) to detect psychiatric disturbances; the Munich Alcoholism Test (MALT
19) to assess alcohol abuse; and the Daily Activity List (DAL
20) to measure physical condition based on the capacity to perform a variety of daily activities that is normal for people in good physical health. The day before surgery, patients filled in the Profile of Mood States (POMS
21), consisting of the subscales Depression, Anger, Fatigue, Vigor, and Tension.
Days 2 through 5 postoperatively, mental status examination and chart review were done for the presence of delirium, according to DSM-III-R criteria,
22 by a research psychiatrist (R.C.vdM. or W.W.vdB.) who was blinded to preoperative assessments. Delirium was diagnosed only if symptoms had been present at least 24 hours and not only on the first postoperative day, which was excluded because of the difficulty of distinguishing between true delirium and possible residual effects of anesthesia. Delirium occurring later than day 5 postoperatively was considered not to be directly related to the surgery and was therefore not included. Patients were also rated on the Delirium Rating Scale (DRS
23), the cognitive status being assessed with the MMSE.
17 Duration and type of surgery, length of time on cardiopulmonary bypass (CPB), hypothermia time, lowest nasal temperature, minutes of mean systolic blood pressure (60 mm Hg, generally considered the value below which cerebral hypoxia occurs), and medication during anesthesia were recorded.
Blood Sampling and Biochemical Analyses
Blood sampling was carried out between 8:00 and 10:00
a.m., just before anesthetics were given. Albumin concentration and levels of cortisol and thyroid hormones were determined as measures for physical condition and physical stress. As a measure for physical condition, we used the ratio of inactive reverse T
3 (rT
3) to active T
3.
13,24 Plasma amino acids (Trp, Tyr, Val, Phe, Leu, and Ile) were analyzed by high-performance liquid chromatography (HPLC).
25 The assay coefficients of variance for the amino acid determinations were below 4%, except for methionine, which was 4.6%. To examine the interdependent relationship of the amino acids, we calculated plasma ratios of Trp, Tyr, and Phe by dividing the plasma concentration of each of these by the sum of the five oLNAA. Plasma ratios are used as peripheral indices of the transport of each of these amino acids from blood to the brain.
26,27 Thiamin, pyridoxine, folic acid, and cyanocobalamin were measured because of their major importance in the metabolism of most amino acids. On the first postoperative day at 8:00
a.m., again, blood samples were drawn for measurement of albumin concentration and levels of cortisol, thyroid hormones, and amino acids.
Statistical Analyses
The Kolmogorov-Smirnov goodness-of-fit test was used to test for normal distribution of all continuous variables. Independent-sample t-tests on all variables that were approximately normally distributed and Mann-Whitney tests on all other variables were used to compare the means and mean ranks, respectively, of delirious and nondelirious patients. Chi-square tests were done for comparison of proportions. Two-tailed probabilities were applied throughout. To compare delirious and nondelirious patients, we performed repeated-measures multivariate analysis of variance (MANOVA) with the between-subject groups factor Subject Type (delirious/nondelirious) and the within-subject groups factor Time (preoperative/postoperative), with regard to the following: plasma amino acids; the ratios of Trp/oLNAA, Tyr/oLNAA, and Phe/oLNAA; albumin; cortisol; and thyroid functions. We then performed repeated-measures ANOVA for individual variables.
RESULTS
For all patients, the incidence of delirium was 13.5% (95% confidence interval [CI]: 10%–17%); for patients under the age of 60 years, the incidence was 0% (95% CI: 0%–4%); and for patients of 60 years and over, the incidence was 20% (95% CI: 15%–26%). Delirious patients were significantly older (
t=–5.23, df=294,
P<0.001). Preoperatively, no significant differences (
P<0.05) between delirious and nondelirious patients were found in cognitive function, existence of psychiatric disturbances, alcohol abuse, functional status, or mood, according to the test instruments used. The scores on the DRS for delirious and nondelirious patients are shown in
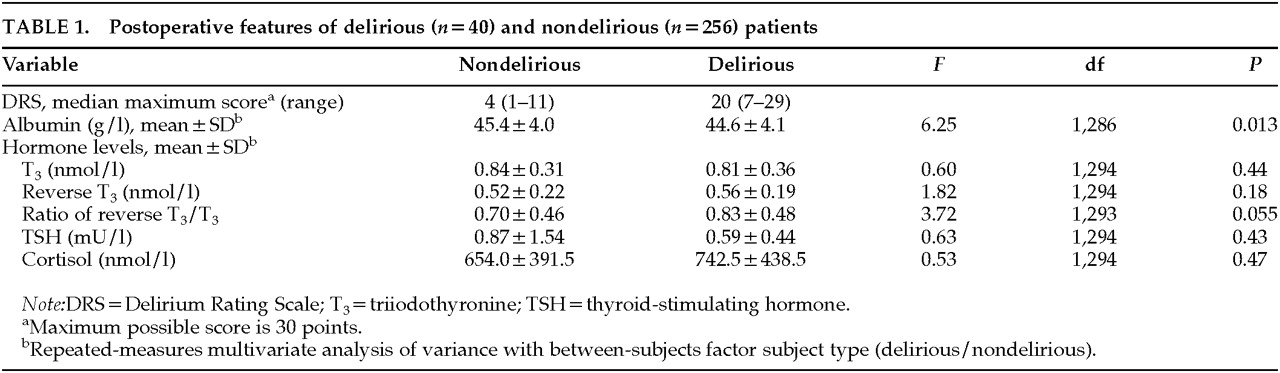
Table 1.
Surgical and Anesthesia Characteristics
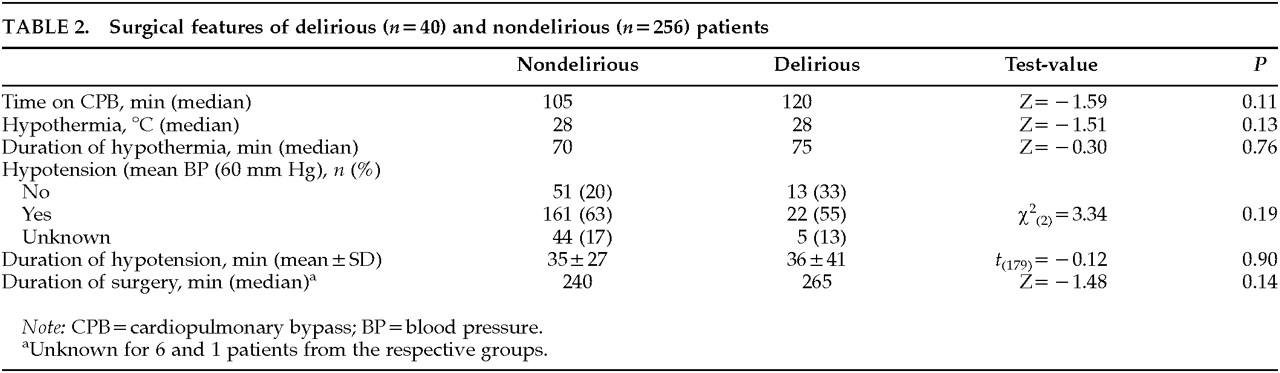
Delirium was not related to the type of surgery (χ
2=2.94, df=3,
P=0.40) Also, neither intraoperative features (
Table 2) nor medications used for anesthesia differed significantly between delirious and nondelirious patients.
Amino Acids
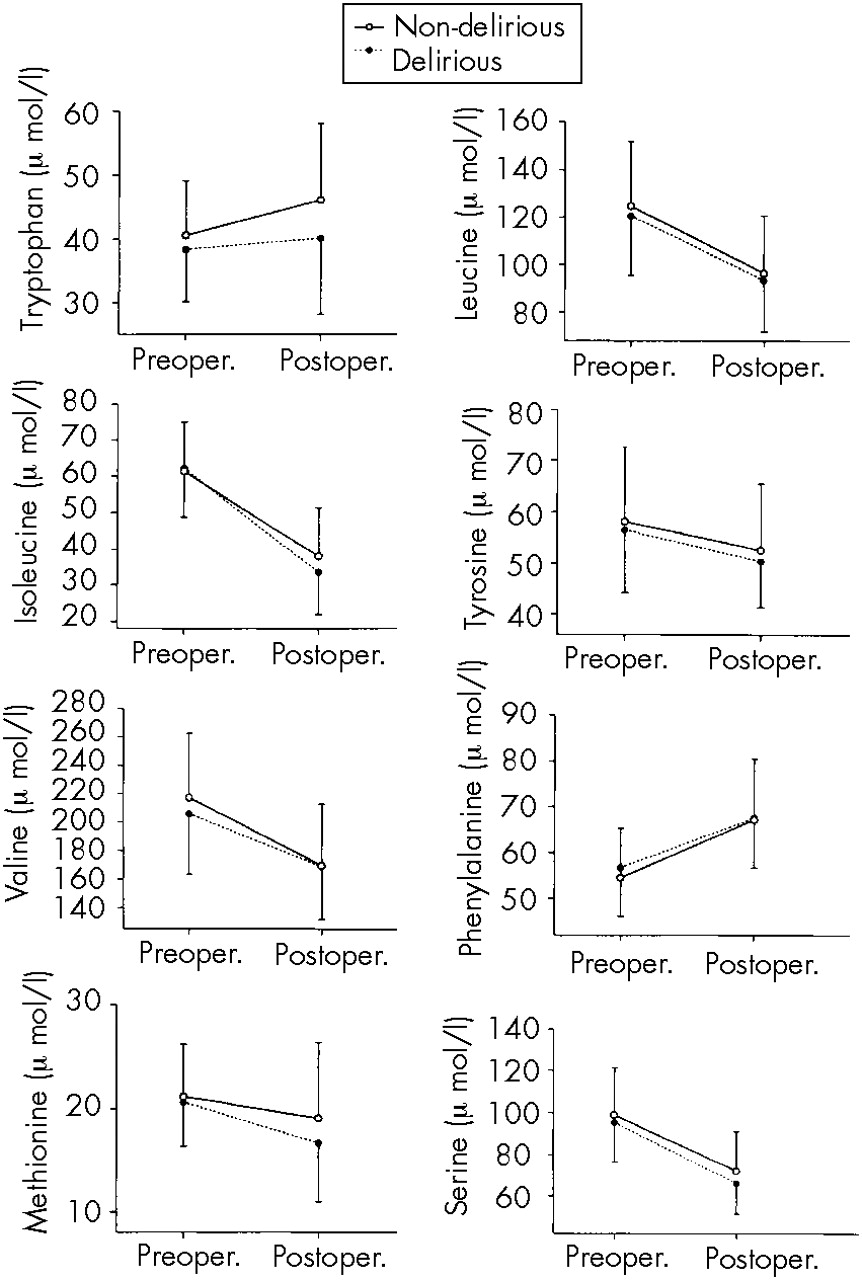
Repeated-measures MANOVA indicated that over time of surgery, in both delirious and nondelirious patients, plasma amino acids decreased significantly (
F=100.66, df=286,
P<0.001) with the exception of plasma Trp and Phe, which increased significantly for all patients (
F=13.40, df=1,294,
P<0.001; and
F=137.51, df=1,294,
P<0.001, respectively;
Figure 1).
There was a significant difference in the postoperative plasma amino acid concentrations between delirious and nondelirious patients (F=2.76, df=286, P=0.004). Repeated-measures ANOVA for individual amino acid differences revealed that this effect was caused mainly by Trp which was significantly less increased in delirious patients than in control patients (F=8.08, df=1,294, P=0.005). However, delirious patients did not respond differently to cardiac surgery (F=1.67, df=286, P=0.096), meaning that the extent of within-group change between the pre- and postoperative amino acid concentrations was similar for delirious and nondelirious patients.
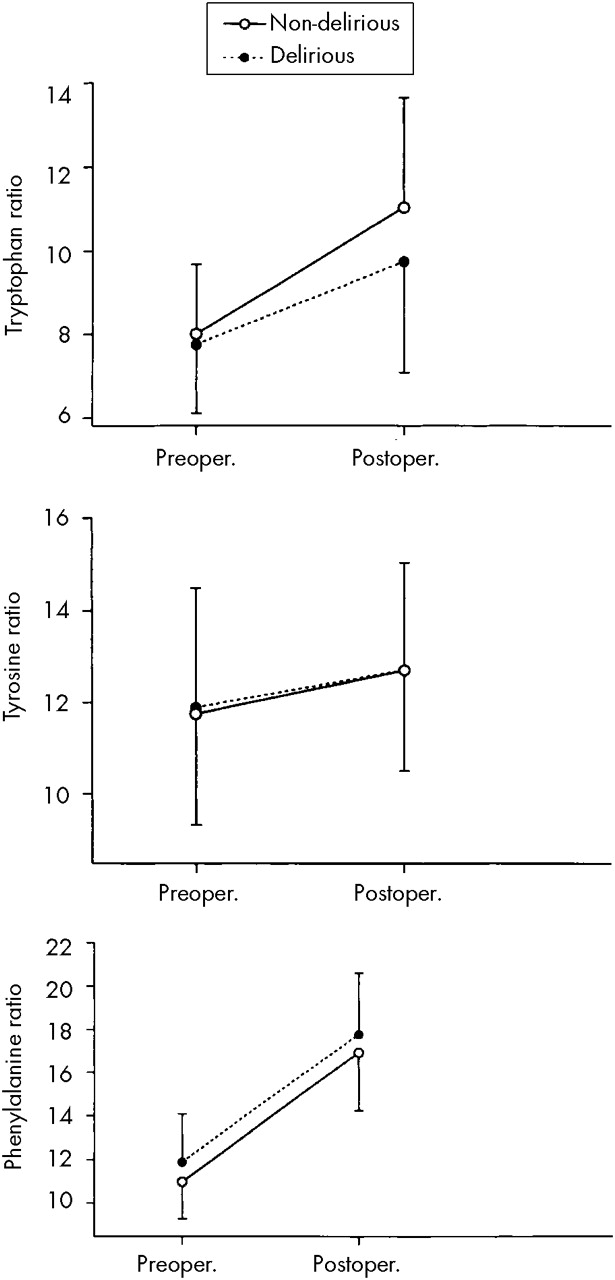
Plasma Ratios of Amino Acids in Delirious and Nondelirious Control Patients
Over time of surgery, the plasma ratios of Trp/oLNAA, Tyr/oLNAA, and Phe/oLNAA increased significantly in both delirious and nondelirious patients (F=151.33, df=290, P<0.001). However, repeated-measures ANOVA showed that the Trp/oLNAA ratio increased significantly less in delirious patients than in nondelirious patients (Time×Subject Type interaction; F=5.72, df=1,294, P=0.017).
When the groups were compared, delirium was associated with different postsurgical plasma amino acid ratios (
F=4.56, df=290,
P=0.001), mainly because delirious patients compared with control patients showed smaller increases in the ratio of Trp/oLNAA (
F=6.62, df=1,294,
P=0.011) and larger increases in the ratio of Phe/oLNAA (
F=5.30, df=1,294,
P=0.005). Apart from the Trp/oLNAA ratio, there was no Time×Subject Type interaction in the between-groups comparison (
F=1.67, df=290,
P=0.14;
Figure 2).
Albumin Concentrations
Not surprisingly, plasma albumin concentrations increased significantly (
F=83.77, df=1,286,
P<0.001) in both delirious and nondelirious patients over time of surgery, considering that they all received albumin and blood substitute transfusions keeping blood at a constant hematocrit value of 20 mmol/l during CPB. Although delirium was associated with a lower albumin (
Table 1) due to the lower preoperative level of albumin in delirious patients, the increase over time of surgery was not significantly different for delirious and nondelirious patients, suggesting that both groups received about the same amount of albumin during surgery.
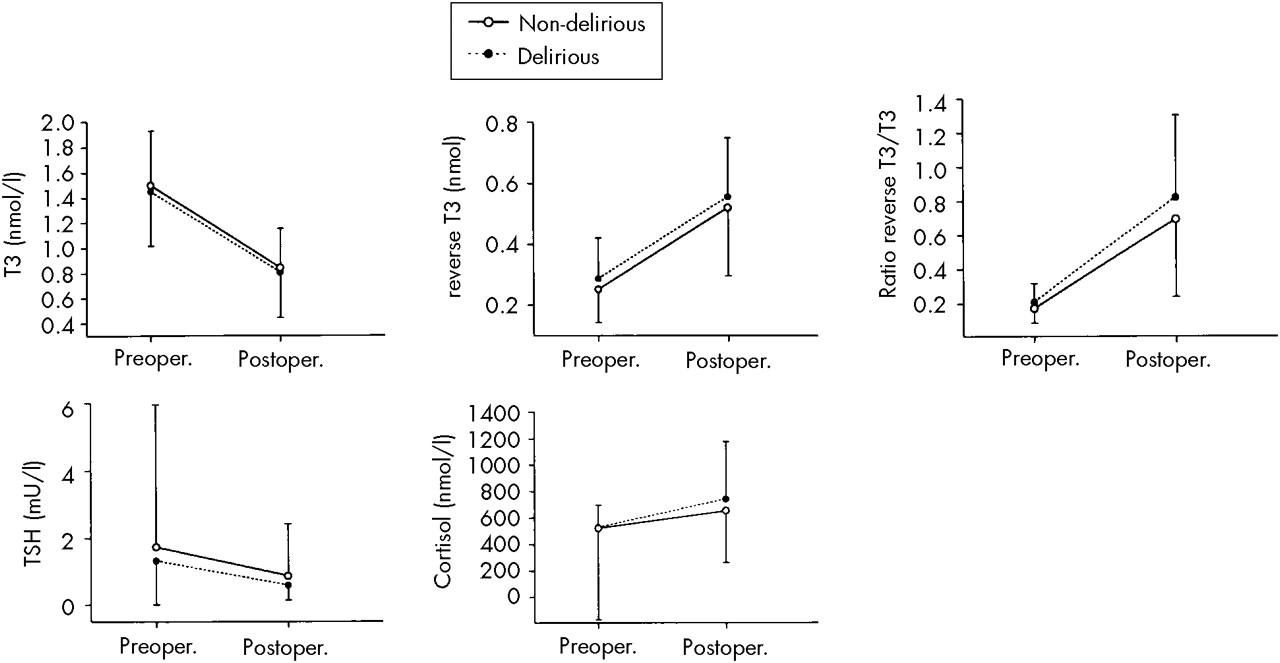
Hormone Levels
In all patients, T
3 and TSH levels decreased significantly (
F=399.68, df=1,294,
P<0.001; and
F=9.53, df=1,294,
P=0.002, respectively), and rT
3 and cortisol levels increased significantly (
F=312.47, df=1,294,
P<0.001; and
F=7.12, df=1,294,
P=0.008, respectively) over time of surgery (
Figure 3).
Although the rT
3/T
3 ratio increased for all patients (
F=239.69, df=1,293,
P<0.001), the delirious patients were in a poorer physical state, as shown by a higher, though not significantly higher, postoperative rT
3/T
3 ratio (
Table 1). Nevertheless, delirious patients did not respond differently to surgery (
F=1.59, df=1,293,
P=0.21). No significant differences between delirious and nondelirious patients were found in their hormone levels (
Table 1).
DISCUSSION
In 296 patients undergoing cardiac surgery, we examined plasma levels of amino acids and physical condition (as apparent from albumin, cortisol, and thyroid hormone concentrations) in relation to postoperative delirium. In this study, the incidence of delirium was low and was not associated with any intraoperative or anesthesia characteristic. The relatively low incidence compared with past reports may be explained by improved surgical and anesthesia techniques.
28In both delirious and nondelirious control patients, almost all amino acids decreased over time of surgery. Contrary to our expectations, plasma levels of Trp and Phe increased for all patients during surgery, although the increase in Trp was less for delirious patients. The plasma elevation of Phe may be explained by decreased conversion into its metabolites due to the hypothermia (28°C) during cardiopulmonary bypass. The metabolizing key enzyme in the liver, Phe hydroxylase, needs tetrahydrobiopterin as a cofactor—and the latter, in the presence of hypothermia, diminished metabolism, and a low T
3 syndrome, may be reduced secondary to a decreased production of ATP.
29 Deficient tetrahydrobiopterin may also underlie the increased plasma levels of Trp because of its cofactor role for the enzyme Trp hydroxylase. Whether the postulated peripheral reduction in the cofactor tetrahydrobiopterin can be extrapolated to the central nervous system is a matter of discussion. Differences in affinity of the central hydroxylating enzymes for tetrahydrobiopterin cannot be ruled out.
Although Trp increased for both patient groups over time of surgery, plasma Trp and Trp/oLNAA ratios were lower in delirious patients than in control patients, in line with previous findings.
10,11 Furthermore, delirious patients showed a higher ratio of Phe/oLNAA; this higher Phe ratio may also hamper their cerebral Trp uptake from blood, since Phe has a particular affinity for the blood–brain barrier in preference to the other amino acids.
27According to their decreased albumin level and increased rT
3/T
3 ratio, delirious patients were in a poorer physical condition than control patients. In line with the findings of Naber and Bullinger,
30 cortisol levels increased during surgery, demonstrating the stressful nature of this major operation, although not differently so for delirious and control patients. This finding is in contrast with the results of a preliminary study by McIntosh et al.,
31 who found a relationship between delirium after noncardiac surgery and increased levels of cortisol. A more sophisticated method of measuring cortisol, such as post-dexamethasone cortisol levels or repeated measurements for a longer perioperative period, might have yielded different results than we obtained, indeed discriminating delirious and nondelirious patients according to their cortisol and stress levels. However, we found no significant differences between delirious and nondelirious patients in their cortisol levels, and therefore a stronger induction of the enzyme Trp pyrrolase in delirious patients cannot be held responsible for the lesser increase of Trp in delirious patients.
Both delirious and nondelirious patients showed a significant decrease of TSH over time of surgery, paralleling the decrease in T
3 hormone level. This is in contrast with the findings of Naber and Bullinger,
30 who reported unaffected serum levels of TSH postoperatively. This decline in TSH may be the result of the inhibiting effect of increased cortisol on the conversion of thyrotropin-releasing hormone in TSH, thus preventing stimulation of the thyroid and maintaining a low-T
3 syndrome.
Our findings are suggestive of changes in cerebral amino acid availability from blood, and of poor physical condition, in delirium after cardiac surgery. The decreased Trp and increased Phe availability from blood for delirious compared with nondelirious patients may give rise to alterations in the cerebral neurotransmitters 5-HT, dopamine, and norepinephrine, threatening adequate cerebral function. The resulting decreased serotonergic function and the normal or increased dopaminergic and noradrenergic function may be contributing pathogenetic factors in delirium after cardiac surgery. This reasoning would suggest that supplementing patients with tryptophan as the precursor for serotonin and blocking the effects of excess release of dopamine and norepinephrine might diminish the risk for delirium.
ACKNOWLEDGMENTS
This work was presented in part at the 8th international meeting on tryptophan research (ISTRY-95), Padua, Italy, June 25–29, 1995.