Pedophilia and Temporal Lobe Disturbances
Abstract
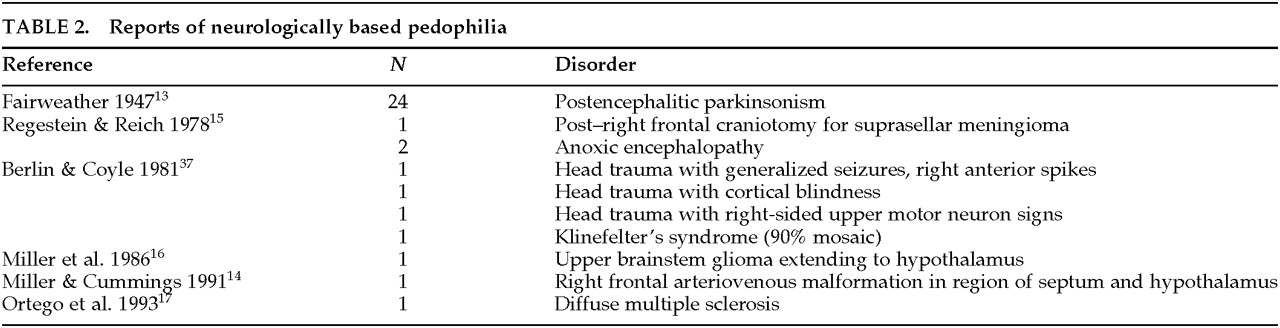
CASE REPORTS
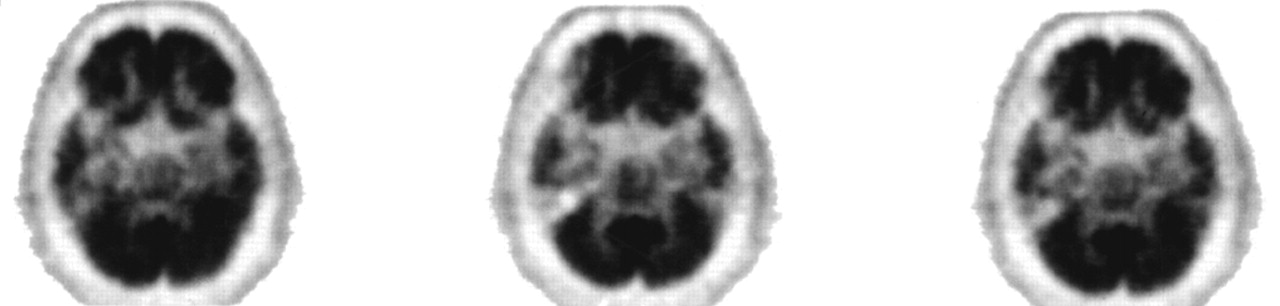
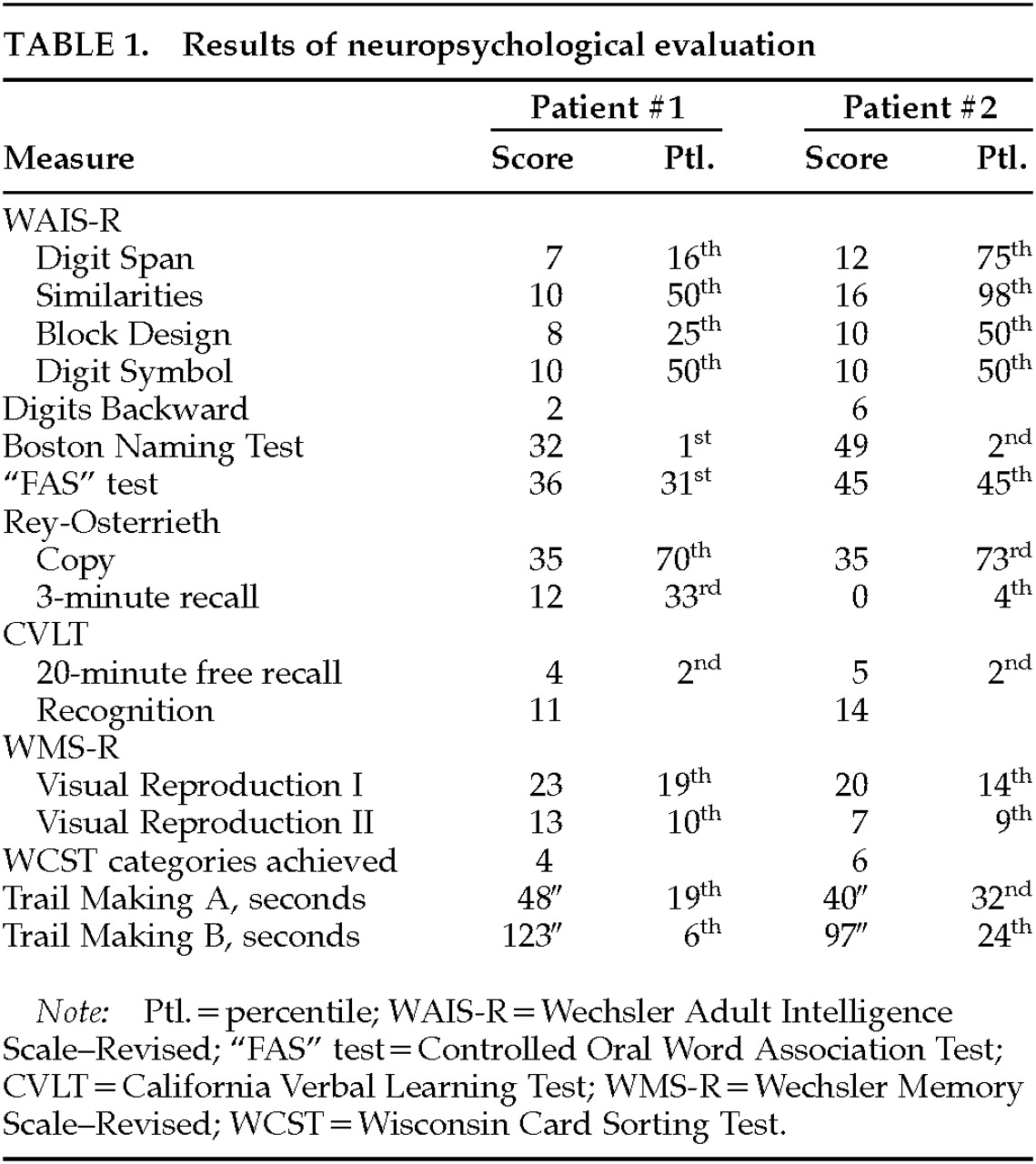
Patient 1. The patient is a 60-year-old left-handed male hospitalized after stalking, accosting, and attempting to molest children. For 18 months he followed children home from school in his car and tried to touch them. At one point, he put his arm around a 10-year-old boy and then struck him when he tried to pull away. He stood at the foot of a pool and leered at the boys, and he exposed himself in front of his neighbor's children. His family was concerned that he would molest the children and would be arrested. The family felt that he had a pronounced increase in sexual activity and in conjugal demands.The patient had had a decline in memory and personality over the preceding 4 years. His personal appearance had deteriorated; he wore the same clothes every day and stopped showering. He intruded into others' conversations and walked into offices that he had no business entering. He constantly pilfered items. In restaurants, he would fill his pockets with sugar packets and napkins, and at one point he had a collection of 28 umbrellas that he had taken from others. His eating behavior had changed as well, and he was eating indiscriminately, taking others' food and even going through and eating from the garbage. He also compulsively took photographs of the sunset every night. Over the preceding weeks he had become verbally and physically aggressive. Recently, his driver's license had been suspended because of a hit-and-run accident.The patient was a former college professor who was being divorced by his wife because of his behavior. In extended family interviews and sessions, family members said that a minister might have molested him at age 18 and that he, in turn, had molested his son when the latter was a small child about 35 years before. The son would not discuss or elaborate on this further. The patient's family history was positive for an unspecified dementia in his mother.On examination, the patient denied a sexual interest in children and believed that he was hospitalized because “talking to strangers was not the right thing to do.” He did not endorse hallucinations, delusions, or paranoia. He was irritable and showed utilization behavior and hyperorality during the interview. His basic attention span was normal, and he was oriented to place and time. He was mildly echolalic, with decreased word generation and verbal stereotypies such as “oh my” repeated over and over again. Comprehension for three-step commands and repetition of spoken language were intact, but confrontational naming was decreased. Verbal memory and nonverbal memory were impaired. Simple constructions were normal. His interpretations of proverbs were concrete, and he had marked perseverations but had no difficulty with Luria hand sequences. The neurological examination disclosed normal cranial nerve, coordination, motor, sensory, and reflex results.Neuropsychological testing (Table 1) showed significant memory impairment, less dramatic declines in executive functions, and relatively poor Wechsler Adult Intelligence Scale–Revised subtest scores, given his background as a college professor. Magnetic resonance imaging (MRI) of the brain did not disclose abnormalities. 18-fluorodeoxyglucose positron emission tomography (FDG-PET) imaging, however, showed a prominent area of focal hypometabolism in the right inferior temporal region, with subtler hypometabolism in the left temporal lobe (Figure 1).The patient met Lund and Manchester criteria for frontotemporal dementia3 (FTD), with a personality change characterized as a progressive decline in social and personal awareness and behavioral changes reflecting decreased judgment and compulsive behavior. The patient was started on paroxetine 20 mg qhs, valproate 500/750 mg, and conjugated estrogens 0.625 mg qd. With this medical regimen and increased supervision, the patient had significant behavioral improvement, including a decrease of his sexual interest in children.
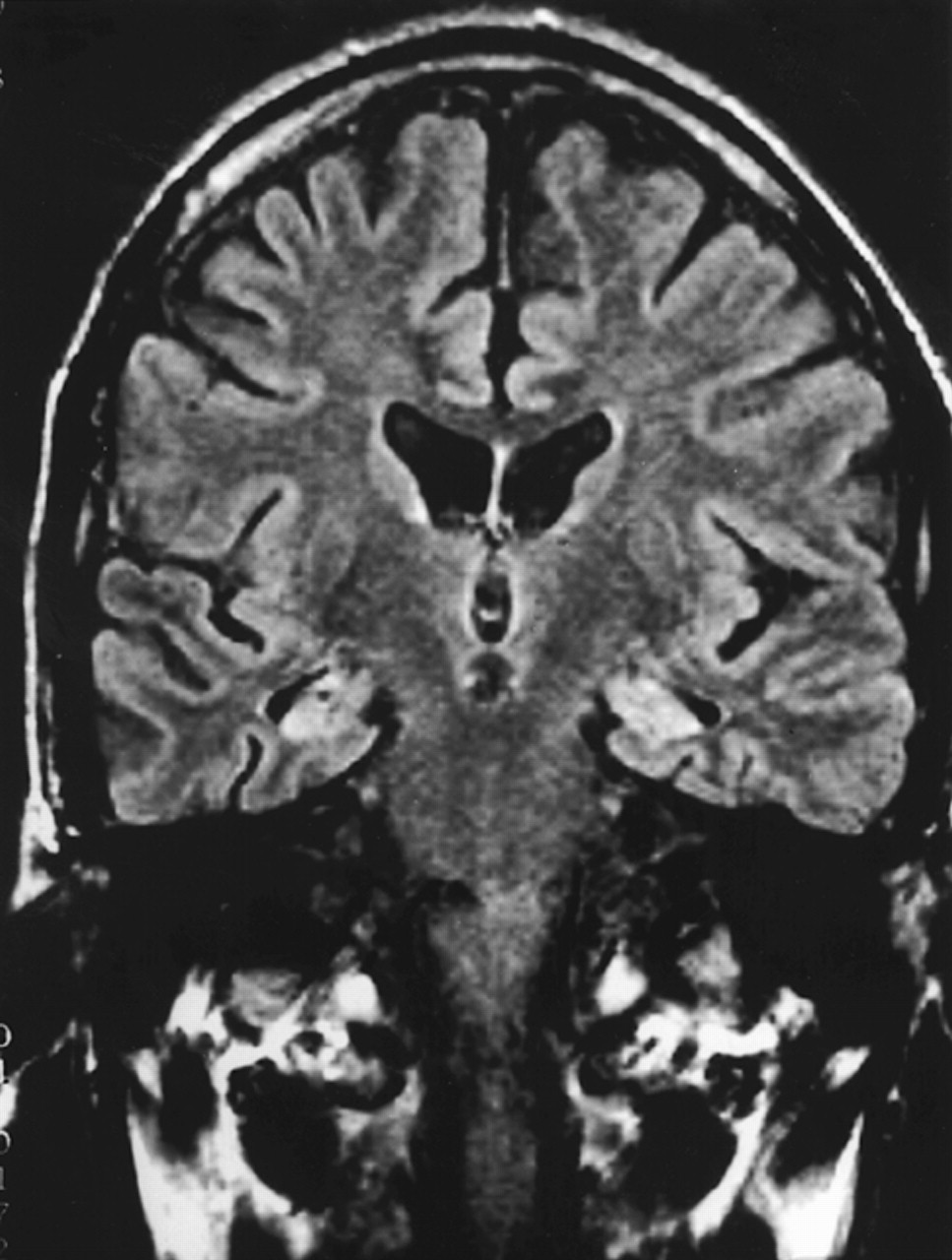
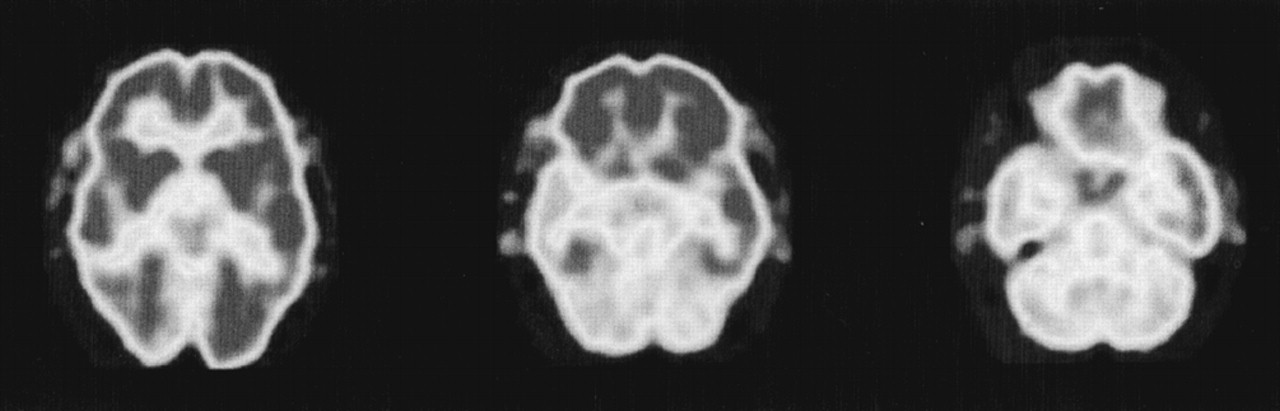
Patient 2. A 67-year-old right-handed artist presented for neuropsychiatric evaluation after spending 18 months in jail for child molestation. He gave an unsolicited “massage” to a 14-year-old boy. He denied sexual intent and claimed to have been demonstrating the technique of pressure point massage. The patient had shown a heightened sexual interest during the prior 2 or more years and constantly sought female companionship. He incessantly spoke of multiple girlfriends, who tended to be much younger than he was.The patient also complained of severe memory difficulty present during the same period of time. He forgot what was told to him, where he left things, and how to get places. He had trouble remembering appointments and forgot to pay his bills. In addition, the patient was depressed. He had suicidal ideation and contemplated using a gas stove or inhalation from his car exhaust to kill himself.His past history was significant for angina pectoris, responsive to sublingual nitroglycerin, and probable cardiac arrhythmias. He had an extensive history of alcohol and drug use, with crack cocaine binges lasting days. As a young man, he was an amateur boxer but never sustained a loss of consciousness. Detailed family interviews disclosed that he was estranged from his grown son. When contacted, the son claimed to have been molested by his father as a child 30 years previously and refused to have anything to do with him now. His family history was otherwise significant for three uncles and one aunt who committed suicide.On examination, the patient was talkative and engaging, with good eye contact and a ready smile. The patient appeared much less depressed than his stated mood. He attempted to flirt with female examiners and at one point exposed himself to a female physician. The patient's thought processes were logical and clear, and he denied any hallucinations or delusions. He was alert and attentive but scored 24/30 on the Mini-Mental State Examination,4 missing the three memory items and three orientation items. Language was fluent, with good comprehension, repetition, and naming. He generated a word list of 20 animals in a minute. Constructional tasks and proverb interpretation were normal. The neurologic examination revealed intact cranial nerves, coordination and gait, and motor and sensory systems. His reflexes were 2+ and symmetrical, with bilateral Babinski responses but negative snout, grasp, or glabellar reflexes.The patient had yearly neuropsychological testing over the next 3 years. His scores on these tests did not significantly change over this time period. He remained with markedly impaired verbal and nonverbal memory and decreased confrontational naming (Table 1). He scored 24 on the Beck Depression Inventory,5 which is in the moderate range of depressive symptoms.Laboratory tests revealed several abnormalities. An electrocardiogram showed occasional premature ectopic complexes. MRI of the head disclosed increased signal intensity and volume loss in the mesial temporal lobes along the hippocampal formations, consistent with bilateral hippocampal sclerosis (Figure 2). The patient had serial PET scans over the 3-year period, which showed a stable area of decreased metabolism in the right temporal lobe (Figure 3).The patient's serial clinical, neuropsychological, and neuroimaging examinations did not indicate a progressive dementing process. Unfortunately, during this period the patient was charged with molesting a 5-year-old boy. His behavior subsequently improved with sertraline therapy and a carefully supervised environment.
DISCUSSION
References
Information & Authors
Information
Published In
History
Authors
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBGet Access
Login options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

