It is now well established that cognitive impairment is a primary feature of schizophrenia and that this may be observed even in patients not treated with neuroleptic medication.
1–4 Dysfunctions in the areas of attention, memory, language, and executive functions have been described in neuroleptic-free patients. However, no study to date has specifically examined procedural learning, a component of implicit long-term memory.
Procedural learning (“learning by doing”) may be defined as the development of skills in which the strategy of execution cannot be explicitly described. Cognitive or motor procedural skills are learned progressively with practice until there is an automatization of the optimal performance. This function is usually assessed with tasks that require unfamiliar motor or cognitive skills. For instance, subjects may be required to learn and replicate an arbitrarily defined visuospatial sequence or to learn to track a moving target along a predictable path. These types of learning were first reported to be impaired in neurological illnesses involving the striatum, such as Huntington's and Parkinson's diseases.
5–7 The striatum was then suggested to be a critical structure involved in procedural learning. In patients with schizophrenia who do not receive neuroleptic treatments, some MRI studies have shown a reduction in the volume of the putamen.
8,9 In addition, extrapyramidal symptoms
10–13 have been described in schizophrenic patients who have never received neuroleptic medication, suggesting a striatal dysfunction.
Given that procedural learning is known to involve the basal ganglia, and that these structures have been found to be abnormal in first-episode patients with schizophrenia,
14,15 one would predict that procedural learning disturbances could occur in these patients. To address this question, we chose to assess the performance of a population of patients with schizophrenia on a procedural task, comparing it with that of healthy control subjects. The goal of this research was to pin down the procedural learning deficits that are due to the disease, free of the potential influence of medication. Drug-naive patients have seldom been tested in the past because of the constraints posed by their condition (e.g., acute illness and necessity of treatment).
METHODS
Subjects
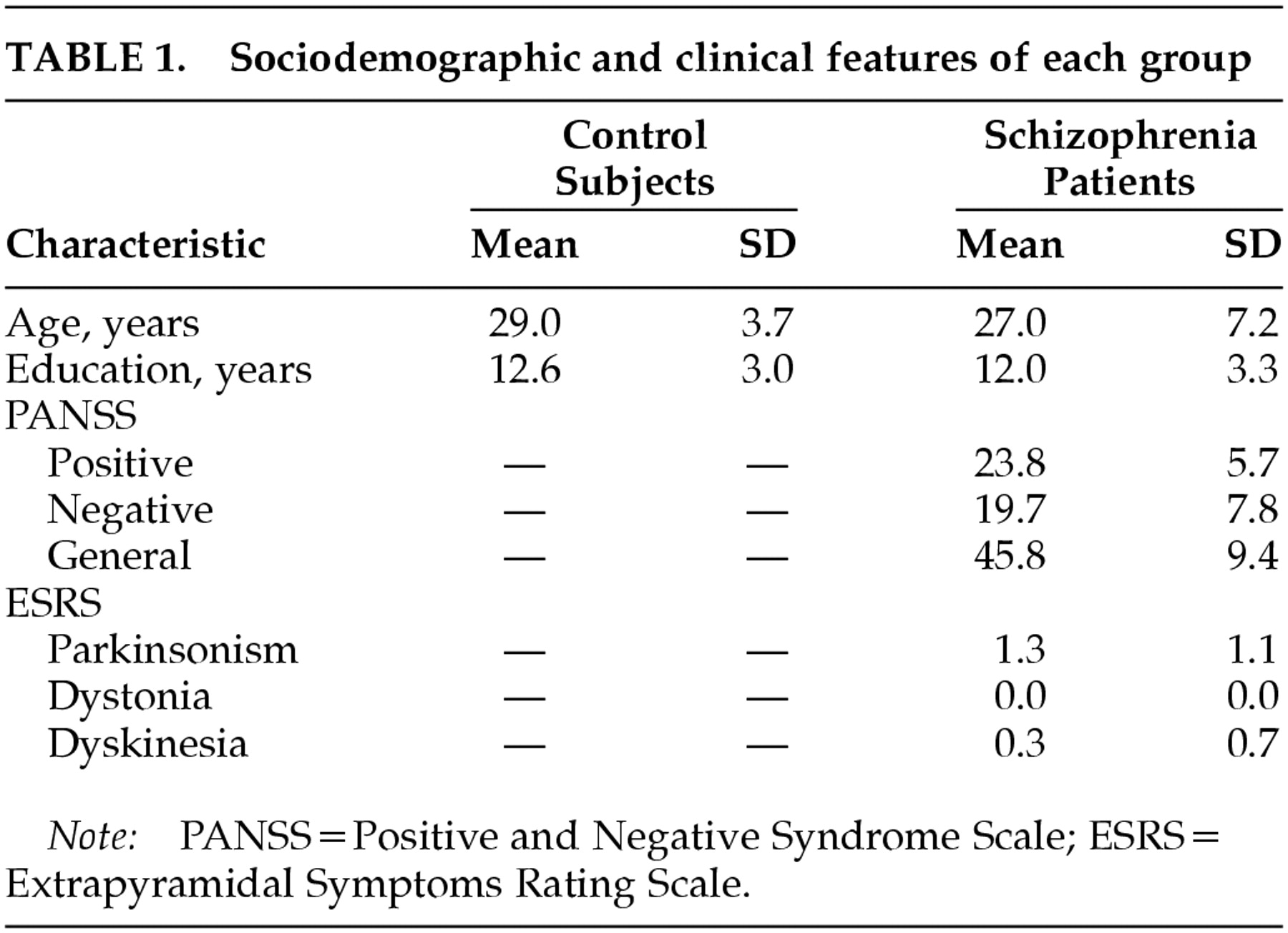
Eleven first-episode drug-naive patients with schizophrenia (8 males, 3 females) were enrolled at the hospital psychiatric emergency and outpatient clinic. They were tested within 1 week of their first visit. All of them were psychotic patients who never received neuroleptic treatment. Exclusion criteria were substance abuse, anesthesia or ECT in the last year, neurological disorder, mental retardation, and any other psychiatric diagnosis. Subjects had to have perfect vision. The diagnosis of schizophrenia was made according to DSM-IV criteria and confirmed by two psychiatrists. Among the included patients, 6 had a prior history of psychiatric illness and 5 presented their first psychotic episode. The diagnosis was reconfirmed 6 months after the current assessment. This procedure has already been used and described by the same team
1 and is similar to those employed in several studies with the same experimental population.
2,8,9,11–13Assessment of clinical symptoms was based on semistructured interviews conducted by trained psychiatrists. The mental status of the patients was scored on the Positive and Negative Syndrome Scale (PANSS).
16 Extrapyramidal symptoms were assessed with the Extrapyramidal Symptoms Rating Scale.
17 Psychopathology ratings were done within 1 week of the cognitive evaluation. The psychiatrists who did the psychopathological evaluations were blind to the results of the neuropsychological testing. A clinical evaluation took about 1 hour. Patients were tested on the procedural task a few days before beginning their medication.
Eleven healthy control subjects (9 males, 2 females) were also enrolled as a control group. The two groups were similarly distributed for age and education. All participants were right-handed. All control subjects were required to be free of neurological or psychiatric conditions. None of the subjects had any diagnosable neurological disease or suffered from substance abuse other than nicotine dependence. Participants were given a complete description of the study, and their written informed consent was obtained.
Procedural Task
The Mirror Drawing Task requires subjects to learn how to move their hands in a new visuospatial context. This task has been frequently used to assess procedural memory in various clinical populations.
18–21 Subjects must draw the outline of a picture (a star) while looking only at its reflection in a mirror, so that proprioceptive information from the hand remains normal but visual information is different from what is usually perceived in everyday life. The task includes a baseline measurement followed by 10 learning trials and a final test measurement. The first five trials (first block of trials) and the second five trials (second block of trials) were separated by a 30-minute pause interval in order to verify if there is a persistence of the optimal performance over time. There was also a 30-minute pause between the second block of trials and the final test measurement (last trial).
For each trial, scores included completion time (in seconds) and number of errors (oversteps beyond the outline of the star to be drawn). In order to avoid a speed/accuracy tradeoff (fast performance with numerous errors in some trials and slow performance with few errors in others), the statistical analyses were run on scores computed according to the following equation: [(completion time)×(number of errors+1)]. To control for basic manual dexterity, each participant was required to draw the same picture (the star) without a mirror before the first trial of the task.
Statistical Analyses
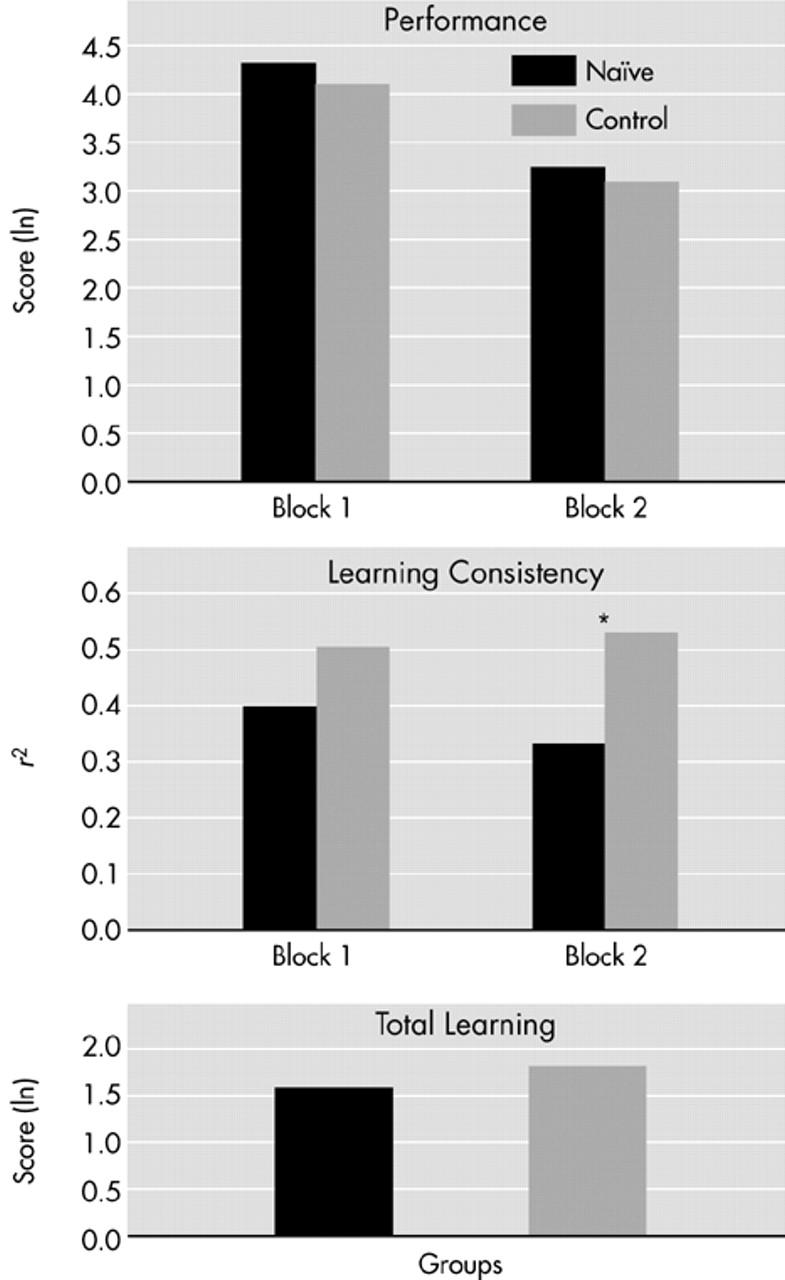
Sociodemographic and clinical measures were compared between groups by using one-way analyses of variance (ANOVAs). For the Mirror Drawing Task, raw data were normalized into their natural logarithm. Three variables were then created and calculated separately for two blocks of trials. The first variable was Average Performance, the mean score for all trials within each block. The second variable was Learning Rate, expressed by the slope of the trial scores within each block. The steeper the slope (values ranging from 0 to 1), the faster the learning rate. The third variable was Learning Smoothness as estimated by the statistical coefficient of determination (r2) for the trials within a block. The greater the learning smoothness, the better the learning consistency; that is, the progressive improvement from trial to trial. Large intertrial fluctuations were therefore reflected by low learning smoothness.
In addition to the three preceding variables, a score for Drawing Without a Mirror was computed [(completion time)×(number of errors+1)] as a measure of basic manual dexterity. Finally, a score for Oscillations, previously described in patients with frontal lobe lesions
18–19 and occurring in our patients, was also compiled at each trial. Oscillations were defined as anarchic, “zigzag,” choppy movements occurring during a trial and corresponding to a temporary inability to move in the correct direction. The number of oscillations in each trial was counted by two independent double-blind scorers. In the event of disagreement between scorers for a given trial, the mean of the two scores was used. Given the distribution of the raw scores, the scores were transformed by using their square roots.
To verify the presence of learning effects in the Mirror Drawing Task, t-tests were performed separately for each group and for each block of trials to see if the slopes differed from zero. Then Average Performance, Learning Rate, Learning Smoothness, Drawing Without a Mirror, and Oscillations were compared between groups by use of one-way ANOVAs.
DISCUSSION
To our knowledge, this is the first time an assessment of procedural learning has been done in first-episode drug-naive patients with schizophrenia. The present study revealed that neuroleptic-naive schizophrenia patients are capable of learning a visuomotor procedural task, despite some degree of abnormality. Learning slopes and general level of performance were comparable to those of the normal control subjects. However, from a qualitative point of view, the learning profile of the patients with schizophrenia was slightly different. The patients were found to have a lower learning smoothness than control subjects; that is, there were more intertrial fluctuations in performance. This difference could not be explained by age, education, or basic manual dexterity, since these control variables were not different between groups.
Considering that the patients in this study were impaired not so much in the degree, but in the quality of procedural learning, one may ask how significant such intertrial fluctuations are from a clinical perspective. Patients with schizophrenia do learn tasks, but they do not benefit as much as normal subjects from the immediately preceding trial. In other words, their learning is less efficient (two steps forward and one step back) than that of normal control subjects (who start each new trial at a level better than the preceding one). From a practical viewpoint, one might visualize learning smoothness in terms of the example of learning to ride a bicycle. After some trials, performances would certainly improve in both normal subjects and patients with schizophrenia. However, while normal subjects would progressively improve their riding from each trial to the following one, schizophrenia patients might occasionally fall and hurt themselves. They both can learn within the same period of time, with the same final performance, but their learning smoothness is not the same. In other words, the harmony of learning is affected in schizophrenia patients when compared with normal subjects.
Procedural learning is crucial to many aspects of daily living, and it seems to have a significant influence on educational and occupational rehabilitation of patients with schizophrenia. The rudimentary employment available to the patients often involves repetitive motor and cognitive sequences that must be progressively developed. A procedural learning disturbance such as the one described in the current study may therefore have some effect in clinical practice or in rehabilitation programs.
Learning smoothness disturbances have also been described in visuomotor procedural learning tasks performed by schizophrenia patients chronically treated with neuroleptics.
21,22 In these patients, the severity of the learning smoothness deficit was found to correlate with the striatal dopamine D
2 receptor occupancy, as measured in vivo with
123I-iodobenzamide.
22 However, the poor learning smoothness described in the present study cannot be attributed to an elevated striatal D
2 receptor occupancy, since these patients have never been treated with neuroleptics. It is therefore possible that an intrinsic striatal dysfunction occurs in these drug-naive schizophrenia patients. This possibility is supported by imaging studies showing a caudate atrophy in these patients.
9,15 In addition, extrapyramidal symptoms, which are usually associated with dysfunctions within the basal ganglia, have been described in such drug-naive patients,
10–13 although these motor dysfunctions were not observed in the patients of the present study.
The lack of intergroup difference in the number of oscillations suggests that the patients' frontal capacity (executive functions) to learn new motor skills remains intact.
18,19 This would not be in accordance with the literature showing that patients with schizophrenia have executive dysfunctions associated with functional or structural impairments of the frontal cortex.
23–25 However, although such executive and frontal dysfunctions have been described in both drug-naive and chronically treated schizophrenia patients, the latter are usually more affected than the former. This difference has been addressed by Gallhofer et al.,
26 who demonstrated that the severity of the executive deficits in schizophrenia increases with the duration of the illness. Oscillations in the Mirror Drawing Task would therefore not be sensitive enough to permit detection of executive deficits in the young drug-naive patients included in the present study. However, in another study performed on chronic institutionalized patients with schizophrenia,
21 this measure was found useful in detecting executive malfunctioning.
Limitations on the interpretation of the findings can be noted. Procedural learning appears as a heterogeneous function that is highly dependent on the sensorial, motor, or intellectual modality involved in the task. It is therefore possible that our results apply only to motor procedural learning. Further studies are needed with other procedural learning tasks involving other modalities.
In summary, procedural learning smoothness may be affected in young drug-naive schizophrenia patients, and this impairment appears to have an early onset, given that it can be measured well before antipsychotic treatment is initiated. In other words, the illness itself seems to account for this feature. Although this learning disturbance does not prevent patients from learning procedures, it may suggest a striatal dysfunction. Our findings concur with other evidence, such as the occurrence of extrapyramidal symptoms and the caudate/ putamen volume abnormalities, that suggests young neuroleptic-naive schizophrenia patients may have a striatal dysfunction.