Psychosis is a devastating feature of many neuropsychiatric disorders, and its pathophysiology remains unknown. While many neurotransmitter systems have been implicated in psychotic illness, dopamine (DA) has a long-standing association with psychosis that remains relevant.
1–7 The role of DA in psychosis is currently supported by several ligand-binding studies in humans.
8–12 Neuroreceptor imaging shows increased striatal DA turnover in schizophrenic patients after low-dose amphetamine trials that is associated with worsening of positive symptoms in patients.
10,12 These findings suggest that exaggerated responses of the DA system may evoke positive symptoms during the acute phase of the illness. The circuitry underlying DA excess in the striatum during acute psychosis remains open to debate. This is due, in part, to the fact that the afferent regulation of DA cells is not fully understood. While the striatum itself is a major afferent of the DA neurons, the amygdala, which plays a key role in emotional function, also sends a projection to the DA system.
13–16 This latter pathway has received little attention. In this article, we review the relationship between the amygdala and the DA system in order to shed further light on the modulation of DA under normal and pathologic conditions.
The DA Cells: Recent physiologic studies show that DA cell firing is linked to the presentation of emotionally salient environmental stimuli.
17,18 DA neurons are activated in response to primary rewards, novel stimuli, and stimuli that become associated with rewards. DA cells also respond to aversive stimuli, albeit in a more heterogeneous fashion.
19,20 Numerous studies show that DA is released in the ventral striatum, amygdala, and prefrontal cortex in response to both rewarding and aversive stimuli,
21–29 which indicates that DA functions in signaling aversive as well as rewarding events. Thus, input from limbic structures, such as the amygdala, may enable the DA cells to respond appropriately to emotionally relevant stimuli.
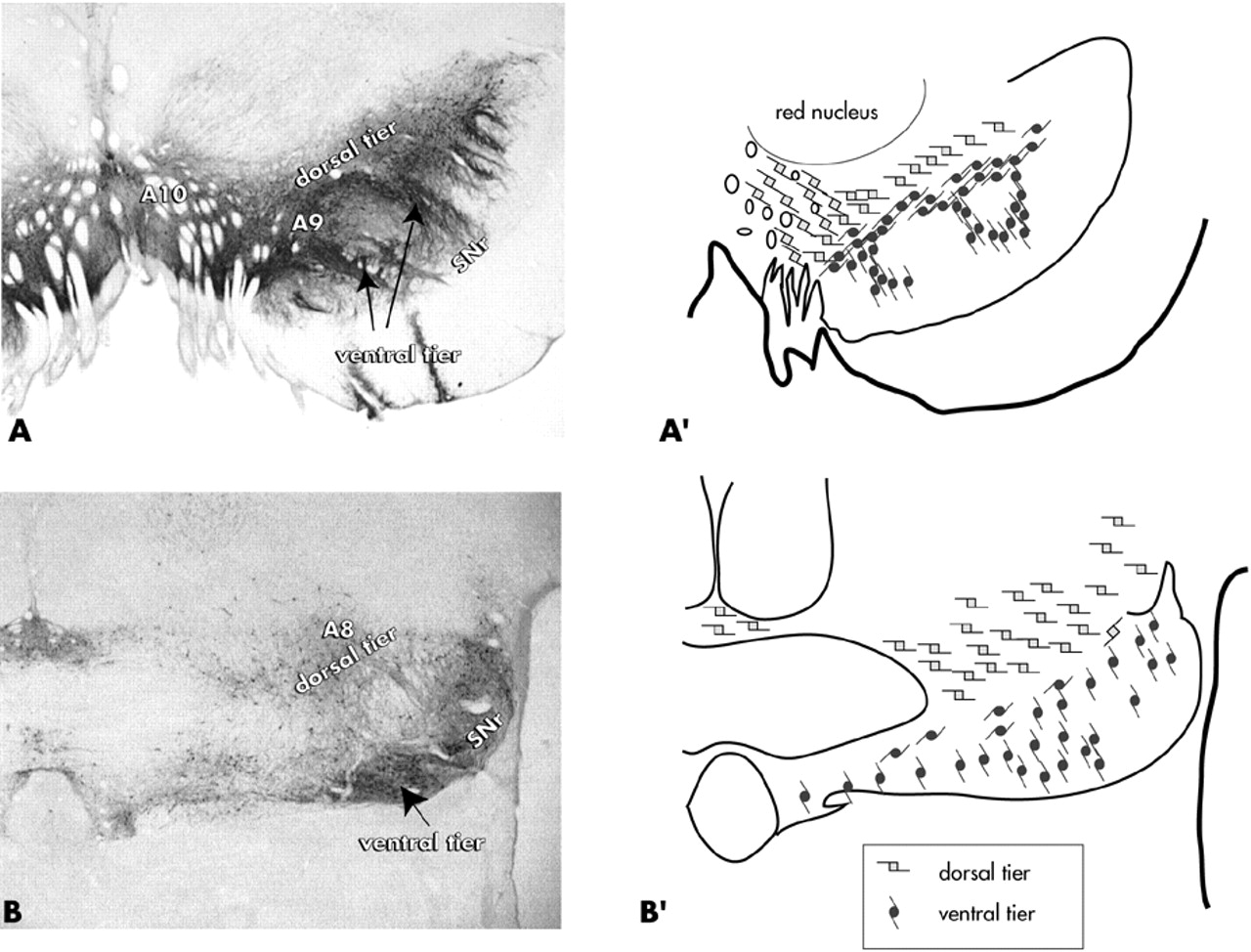
The majority of the brain's DA cells are located in the ventral mesencephalon. Historically, the ventral tegmental area (VTA) has been associated with the “mesolimbic” system, while the main substantia nigra, pars compacta has been considered part of the “nigrostriatal” pathway. More recently, the DA cells have been divided into dorsal and ventral tiers that are based on morphologic, chemical, and connectional properties
30–34 (
Figure 1). In primates, the dorsal tier encompasses the VTA (A10 neurons), the contiguous pars dorsalis, and the retrorubal field (A8 neurons). This continuous expanse of dorsal tier cells is identified by immunoreactivity to calbindin-D28k, a calcium binding protein. The ventral tier neurons include the main “densocellular” part of the substantia nigra, pars compacta and its vertically oriented cell columns (A9 neurons), which are calbindin-negative.
In primates, the dorsal and ventral tiers are distinguished by differential input/output pathways. The ventral striatum is the main limbic input to the dorsal tier,
32–33 which projects back to the ventral striatum, amygdala, and cortex.
33,35–38 While cortical inputs to the DA cells are well described in studies performed on rodents,
39–40 there is not clear evidence to date of a corticonigral projection in primates (review in Haber and Fudge).
41 In contrast to the dorsal tier, the ventral tier is broadly interconnected with the entire striatum through a continuous series of loops
33 and has few outputs to the cortex and amygdala. Thus, the dorsal and ventral tiers have differential input and output channels that partially overlap in the ventral striatum.
The Role of the Amygdala: Given evidence suggesting that the DA cells respond to emotionally salient cues, the role of the amygdala in modulating this system is of key importance. The amygdala is a prominent limbic structure that is essential for linking sensory experience to emotional salience.
42–44 In humans, amygdaloid activation is readily evoked by emotionally valenced facial expressions.
45–49 The dorsal amygdala (corresponding to the region known as the “extended amygdala”) is activated when facial cues switch from neutral to emotional expressions,
47 and with changes in emotional intensity of sensory stimuli.
50–52 Therefore, the extended amygdala may play an important role in detecting shifts in the relative salience of environmental cues, while the “amygdala proper” functions in the emotional coding of sensory stimuli.
The Amygdala and Psychosis: The possible role of the amygdala in psychosis was identified many years ago through studies on stimulation and electroencephalogram (EEG) abnormalities in schizophrenic patients.
53–55 Subsequently, it was reported that direct stimulation of the amygdala in awake humans without schizophrenia elicits a range of complex sensory and emotional states, including fear, anxiety, feeling of familiarity, and complex hallucinations.
56,57 Endogenous abnormal amygdaloid stimulation, which can occur in partial complex seizures, has long been associated with the development of “schizophrenia-like” psychoses.
58–61 Patients with complex partial seizure and psychosis frequently have brain lesions in or near the amygdala.
61–65 The phenomenology of psychotic symptoms underscores the close relationship between the sensory and emotional information that converge in the amygdala. During acute illness, the perception of sensory material deteriorates, along with the interpretation of its emotional relevance. Auditory hallucinations and delusional beliefs are characterized not only by distorted sensory perceptions, but by distinct emotional content or misattribution of emotional meaning to sensory information. Auditory hallucinations, which are experienced as coming from the external world (sensory distortion), are frequently experienced as negatively valenced (emotional content). Consistent with the significant link between emotion and sensory perception, recent studies show that patients with schizophrenia tend to misattribute their own voice to that of another when the voice feedback is slightly distorted or content is derogatory.
66Schizophrenic patients also have difficulty performing tasks that require an evaluation of emotional content of complex sensory stimuli.
67–71 Functional imaging abnormalities of the amygdala at rest have been observed in schizophrenic patients performing such tasks. Such findings, however, are variable across studies.
72–77 The array of abnormal imaging findings across studies may be due to a number of factors, including heterogeneous symptomatology across samples (varying levels of positive and negative symptoms), the variability in probe stimuli and tasks used across studies, and failure to control for age and gender. The current data suggest that there is not a simple under- or overactivation of the amygdala in schizophrenia, but rather a pattern of inappropriate responses compared to controls, reflecting emotional “coding errors.”
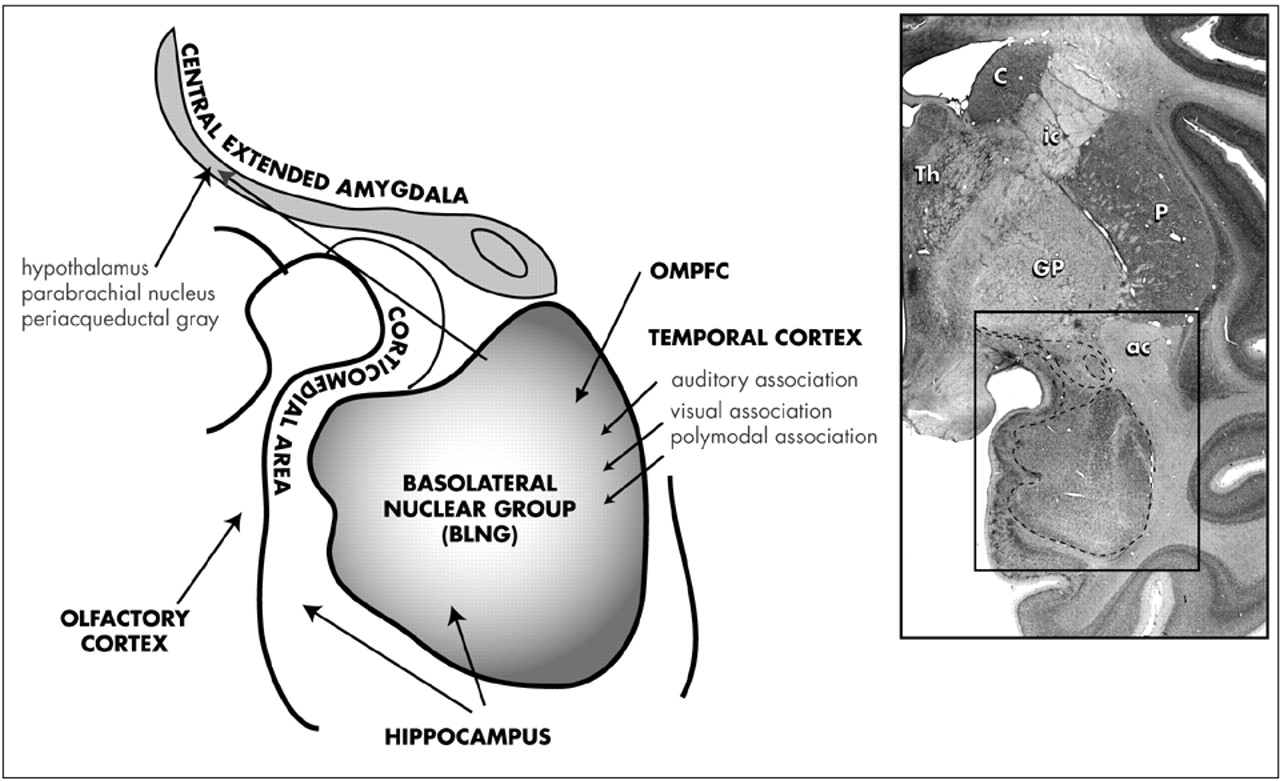
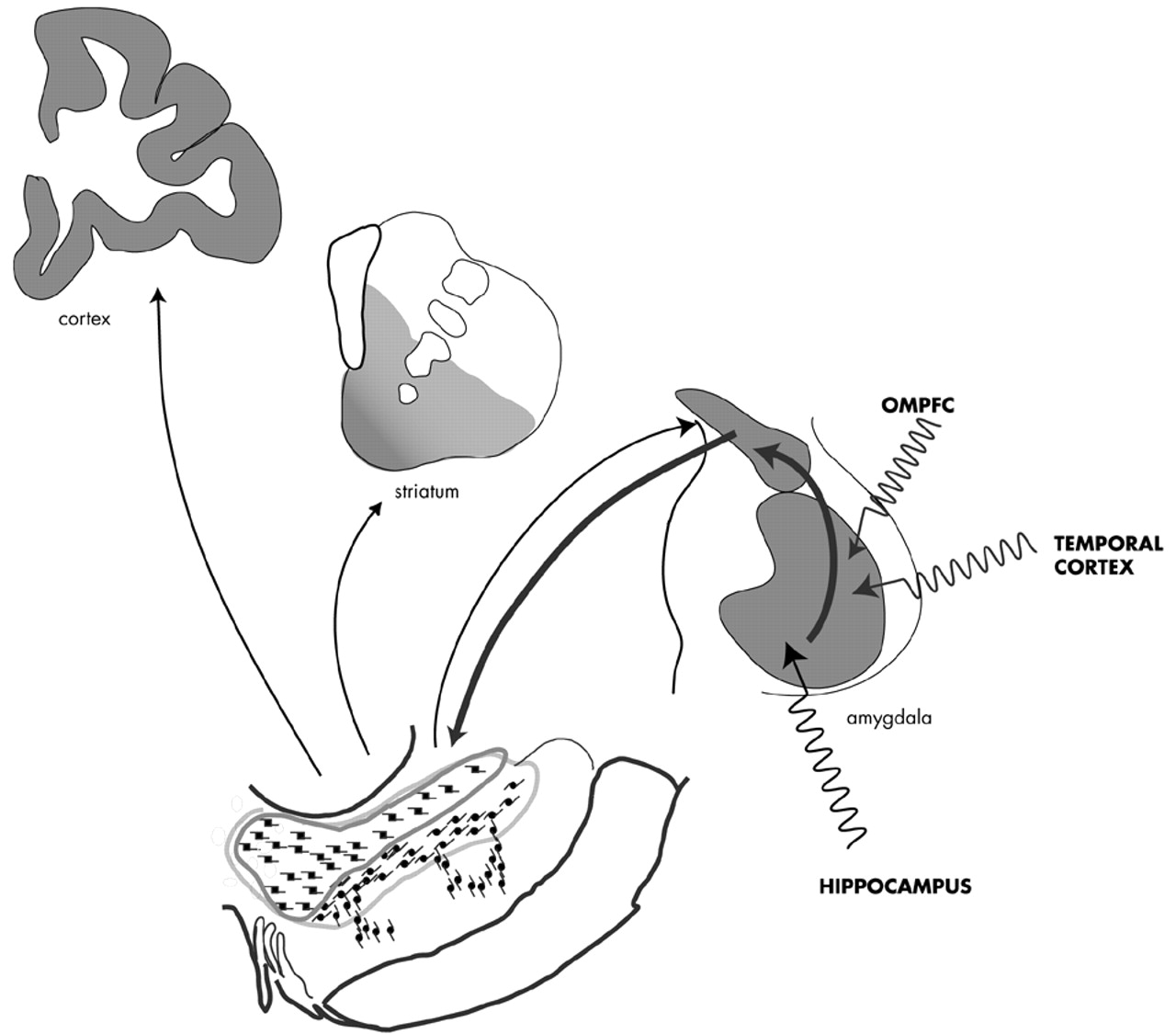
The Amygdala: Anatomy and Pathways: The anatomy of the primate amygdala is consistent with its central role in integrating sensory and emotional information (
Fig 2). The basolateral nuclear group (BLNG) is the main nuclear “receiving” group and receives inputs from the temporal cortex, the orbital and medial prefrontal cortex (OMPFC), and the hippocampus.
78–81 The temporal cortex mediates higher order auditory and visual processing, while the OMPFC is involved in determining the relative value of reward or punishment.
82,83 The hippocampus is vital in the recalling of previously conditioned stimuli.
84 These afferent connections provide the necessary elements for evaluating complex sensory data, with respect to emotional salience. Consistent with this anatomic organization, subsets of BLNG neurons are “tuned” to the affective as well as the sensory dimensions of a stimulus. The firing patterns of these neurons are modified as the animal's experience of the stimulus evolves (i.e., if an unfamiliar object becomes familiar or a previously rewarding stimulus becomes aversively conditioned).
85 Other major amygdaloid regions include the corticomedial amygdala, which receives olfactory and hippocampal inputs,
80,81,86 and the central amygdaloid nucleus (CeN), which has unique connections among other amygdaloid nuclei.
The CeN and Central Extended Amygdala: The CeN stands apart from other amygdaloid regions in that it receives inputs from virtually all the amygdaloid nuclei.
87–89 Additionally, the CeN receives information from the “internal milieu” due to its connections with the hypothalamus and autonomic and visceral centers of the brainstem.
90–94 CeN neurons are distinguished from the rest of the amygdaloid nuclei because they contain a large array of neuropeptides,
95–98 some of which are altered by environmental stress.
99,100 These fundamental differences have lead to a recent debate on whether the CeN actually belongs to the amygdala, or whether it is part of another structure known as the “central extended amygdala.”
101–103 In the concept of the central extended amygdala, the CeN is part of a cellular continuum that spans the basal forebrain to include another region of the limbic forebrain known as the lateral bed nucleus of the stria terminalis (BSTL)
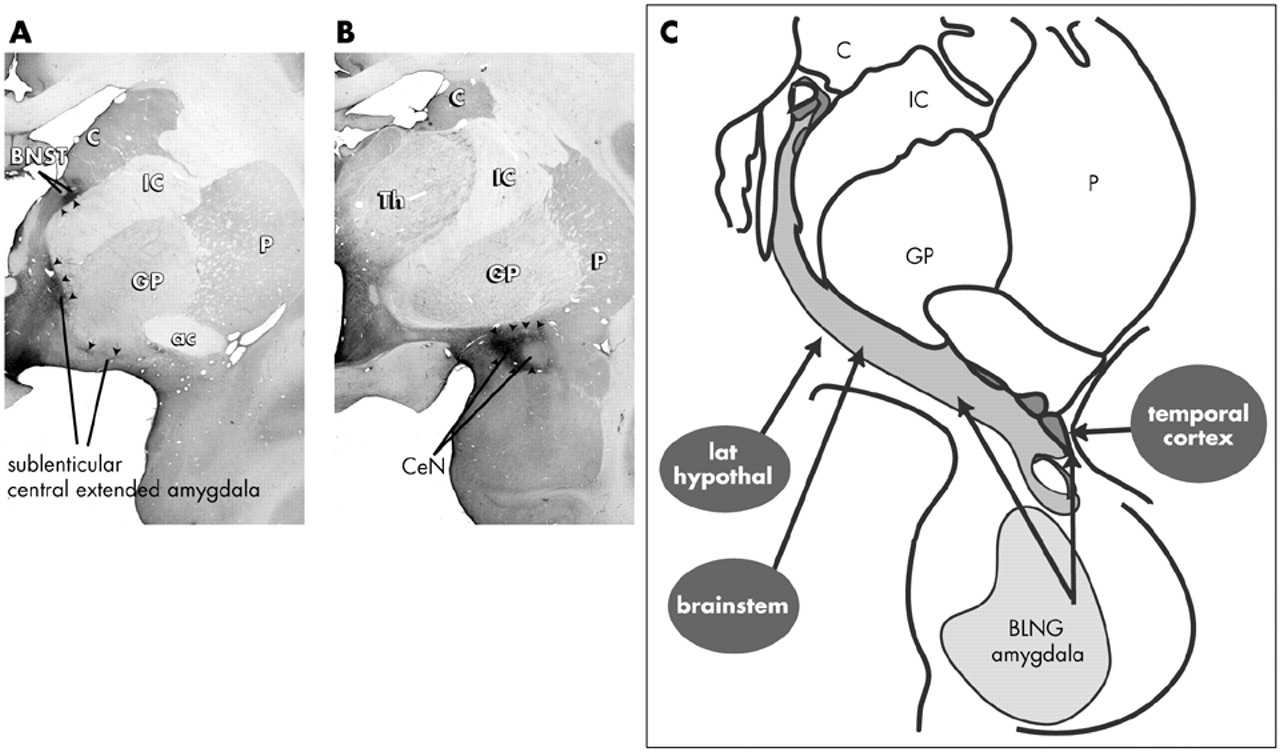
101–104 (
Fig. 3). The concept of the central extended amygdala originated in developmental studies that showed that the CeN and BSTL arise as one structure in the fetus and later are divided into rostral (BSTL) and caudal components (CeN) by the fibers of the internal capsule
105 (for review).
101 Cellular elements remain embedded in the fibers of the internal capsule, forming cell bridges between the two structures. Consistent with a common developmental origin, the BSTL and CeN are symmetrical structures, with respect to their cellular and histochemical organization.
97,106–109 In addition to structural similarities, they share similar inputs, including afferents from the basolateral amygdala and hypothalamus.
110–112 The medial subregion of the central extended amygdala is unique in that it receives converging inputs from both the BLNG and brainstem centers that are involved in visceral and autonomic function.
92,94,113,114 Thus, the medial central extended amygdala is a site where information from the external milieu (via the basolateral amygdala) and internal milieu (via the brainstem/hypothalamus) is combined (see
Figure 3C).
The Missing DA Connection: Amygdalonigral Pathway All three amygdaloid subdivisions project to the ventral striatum
115 and can, as a result, indirectly influence DA neurons through amygdalo-striato-nigral loops. However, the CeN has a direct input to the DA cells. Older studies conducted on rodents before widespread recognition of the extended amygdala concept indicate that the BSTL, which forms the rostral extended amygdala, also projects to the DA neurons (for review see Fudge and Haber).
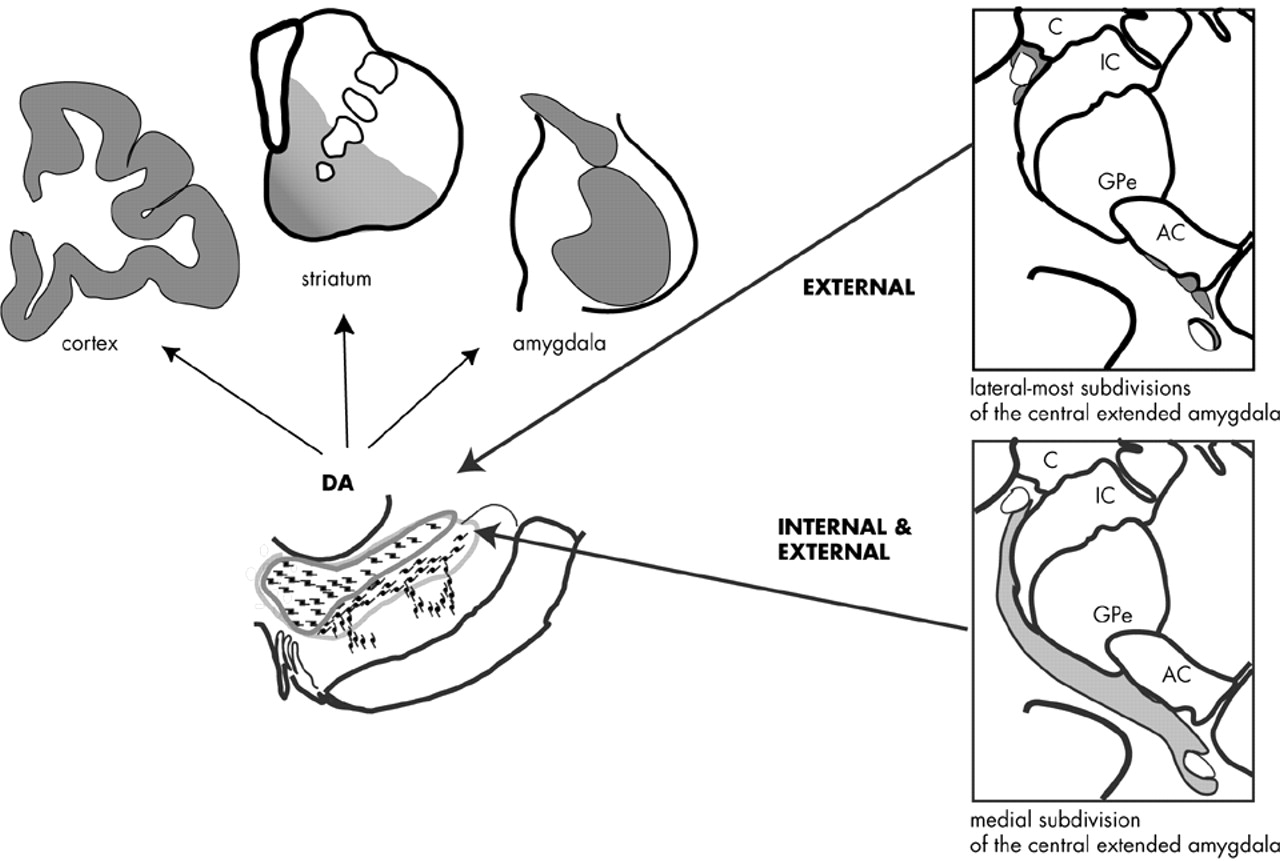
116–117 These anatomic studies suggested that the entire central extended amygdala might function as an important conduit by which emotionally relevant information influences the DA system. In a series of tract tracing studies, we recently examined how the entire central extended amygdala interacts with specific DA subpopulations in the primate. Injections of retrograde tracers placed within specific DA subpopulations resulted in a near-continuous stream of labeled cells through the central extended amygdala. The BSTL and CeN were labeled together in an “all or none” manner so that labeled cells in the CeN were always accompanied by labeled cells in the BSTL and in cellular bridges throughout the basal forebrain. Conversely, injection sites that failed to label one part of the central extended amygdala also failed to label other regions. These observations support the idea of the central extended amygdala as a unified structure that spans the basal forebrain. The central extended amygdala projects most densely to the dorsal tier neurons, particularly those in the caudal dorsal tier (e.g., “retrorubal” group, A8 neurons). The medial subdivisions of the central extended amygdala and lateral subdivisions that surround the lateral core are the source of dorsal tier inputs (
Figure 4). The dorsal part of the ventral tier receives moderate input, but only from the medial subdivision of the central extended amygdala. The ventral cell columns and the pars reticulata receive few, if any, inputs from the central extended amygdala.
In summary, the central extended amygdala influences specific DA subpopulations by two pathways. The medial part of the central extended amygdala, which integrates external stimuli (channeled from the BLNG) and internal cues (from the hypothalamus and brainstem), projects broadly to both the dorsal tier and dorsal portion of the ventral tier. Together, these DA subpopulations project not only to limbic brain regions (the amygdala and ventral striatum) but also to central (cognitive) regions of the striatum and cortex. Converging information from the external and internal environment can, therefore, modulate DA in these diverse brain regions. In contrast, the lateral-most regions of the central extended amygdala project exclusively to the dorsal tier. These lateral extended amygdala regions primarily receive information from external stimuli (via the BLNG and temporal cortex) and are relatively devoid of inputs from the internal milieu. Thus, information about salient stimuli in the animal's external environment is channeled selectively to the dorsal tier neurons, modulating DA in the amygdala, ventral striatum, and cortex.
The Extended Amygdala-Nigral Path: A Direct Link Between the Amygdala and the DA System: The studies above indicate that the central extended amygdala forms a conduit between the BLNG and the DA system. While we have long known of strong inputs from the BLNG to the extended amygdala, the physiology of this connection has only recently been explored. BLNG stimulation results in complex responses in the medial CeN (the caudal portion of central extended amygdala). Detailed analysis indicates that in the medial CeN, neuronal responses depend on both direct glutamatergic inputs from the BLNG and indirect GABAergic input through “intercalated” neurons that lay interposed between the BLNG and CeN. The intercalated neurons receive BLNG inputs and, in turn, inhibit the medial CeN. This arrangement suggests that parallel inhibitory inputs through the intercalated cells “fine tune” excitatory BLNG inputs to the medial CeN neurons. The medial CeN neurons can be activated or suppressed depending on the location, timing, and intensity of BLNG stimulation by this fine tuning mechanism.
119–121 Thus, the DA subpopulations that are modulated by the medial CeN are influenced by firing patterns originating in the BLNG, which are further modulated by inputs from intercalated neurons and brainstem and hypothalamic centers. These additional sources of input to the central extended amygdala strongly suggest that it functions as a higher processing center for information that is channeled from the BLNG.
Relevance to Psychosis: The temporal cortex, the OMPFC, and hippocampus are the main glutamatergic projections to the BLNG, and are major sites of structural abnormalities in the schizophrenic brain
122–131 (see also review by Arnold (
Figure 5).
132 Under normal circumstances, these excitatory inputs shape amygdaloid output.
133,134 Recent data indicate that such excitatory inputs are in a position to permanently change BLNG responses over time.
135–138 Together, these studies indicate that the amygdala is a key site for “emotional learning,” with a high potential for plastic transformation. In schizophrenia, however, these afferent inputs may be structurally impaired, and thus a source of aberrant excitatory signal. Over time, the emotional coding of sensory cues by the amygdala may be compromised due to such faulty inputs. In turn, these “coding errors” can be conveyed to the DA system. Disruption of the DA regulation is hypothesized as a key factor in maintaining psychotic symptoms by reinforcing or sensitizing aberrant brain circuitry.
139–140 The BLNG-extended amygdala-nigral path is one way that impaired BLNG signals can dysregulate DA firing patterns over time. Although the physiologic and chemical properties of the extended amygdala-nigral portion of this pathway are far from understood, DA output is affected when afferent signals that originate in the amygdala are impaired.
141,142 Furthermore, the DA neurons show complex responses following CeN stimulation, some of which have complex sequences and long durations.
143,144 Since the CeN and entire central extended amygdala are characterized by a large number of peptides and transmitters, it is important to delineate how the specific chemical features of this pathway influence DA cell firing in both acute and chronic settings.
CONCLUSION
While the amygdala is almost certainly involved in the emotional coding of environmental stimuli, its precise role in the development of psychotic symptoms remains to be determined. The plasticity of the amygdala and its role in emotional learning suggest that its circuitry is constantly modeled over development. This implies that normal amygdaloid function may be different in childhood and adolescence, the period in which psychosis typically emerges.
51 More studies on normal amygdaloid function across the developmental timeline are needed if we are to pinpoint the deterioration of sensory/limbic processing in psychosis. Additionally, the way in which DA is influenced by normal and abnormal amygdaloid signals—both acutely and over time—must be studied further. The peptide and transmitter content of the central extended-amygdala DA pathway also warrant more investigation. Physiologic studies, which detail responses of the DA system to extended amygdala stimulation both acutely and over time, will be useful in understanding the particular function of this path. More detailed elaborations on the link between the amygdala and the DA system will broaden our understanding of the pathophysiology of psychosis and provide another important piece of the DA puzzle.
ACKNOWLEDGMENTS
This was work is supported by MH63291 and the Leonard F. Salzman Award (J.F.).