Neuroanatomical sites of
a priori significance for the regulation of ACTH and cortisol during sadness include the amygdala,
9 hippocampus,
10 and the medial and subgenual PFC.
3,11,12 Neuroanatomical sites of
a priori significance for sadness (independent of neuroendocrine measures) would also be expected to affect these regions,
13,14 but further recruit a distributed network of structures, including the dorsolateral PFC, orbitofrontal PFC, and insular and inferior parietal cortices.
13–15 This study, therefore, has the primary objective of identifying the neuroanatomical regions of relevance to regulation of the HPA axis during sadness (i.e., the SPM endocrine covariate analysis) and a secondary objective of replicating both the neural correlates of sadness independent of neuroendocrine measures and the endocrine response to sadness independent of the assessment of brain activity.
DISCUSSION
The investigation of mood from the perspective of a paradigm that more directly integrates psychoendocrinology and functional neuroimaging holds significance for understanding the neurobiology of the basic emotions and for furthering an understanding of the pathophysiology of some psychiatric illnesses (e.g., mood disorders). Our study concurrently investigated the endocrine and neural correlates of induced sadness by means of collecting endocrine measures at time points bracketing the temporal window of radiotracer injection, thus allowing for an integrated evaluation of these two neurobiological domains (ones that typically have been investigated in a manner whereby endocrine measures are not closely temporally correlated with cerebral activity).
Functional neuroimaging studies of induced sadness are beginning to demonstrate some degree of consensus. Our findings of greatest
a priori interest (for sadness versus neutral induction, without investigation of the neuroendocrine measures) include increased rCBF in the left OFC, right ACC, and right insula—sites that have been identified, in part, by other investigators.
13,15 To our knowledge, post hoc findings observed in this study, which include increased rCBF of Broca’s area, the left middle and superior temporal gyrus, and decreased rCBF of the left anterior temporal pole and occipital gyrus, and anterior cerebellum bilaterally, have not been observed as reliably by other investigators. Although not identified in our study, rCBF changes in the dorsolateral PFC, medial PFC, and medial temporal cortices have been observed in several different studies.
13,15,33,34 Thus, integration of our results with findings from other studies suggests that a highly distributed network of cortical and subcortical structures mediates the experience of sadness.
The neural correlates of sadness, however, are merely one aspect of the complicated neurobiology of this emotion. Unification of neuroimaging and endocrine paradigms allows for in vivo correlation of hormone levels with neural activity, specifically in networks that are completely intact. Much of the research that combines neural and endocrine techniques is currently conducted on animals, where a discrete lesion is placed in the neuroanatomical region of interest, with subsequent assessment of change in cortisol levels. A possible shortcoming of lesion paradigms is their inability to assess the remote network effects of the “discrete” lesion. A lesion in a discrete portion of the PFC may have significant reverberations throughout the interacting cortical and subcortical network, of which the lesioned region is merely one component. For example, it has been demonstrated that lesions of the medial PFC and ACC alter cortisol responsiveness in rats exposed to stress.
3,4 It is well established that cortisol secretion from the adrenal gland is partly dependent upon ACTH release from the anterior pituitary, which is itself dependent upon CRF release, of which the paraventricular nucleus (PVN) of the hypothalamus is a major site of production.
10,35 Activity of the PVN is thought to be regulated by several neuroanatomical regions: the amygdala and bed nucleus of the stria terminalis are stimulatory, while the hippocampus is inhibitory.
10 Although the medial PFC and ACC also appear to regulate the PVN, it is unclear to what degree these regions do this directly or through interaction with other neuroanatomical structures. For example, it is possible that a lesion of the medial PFC may (inadvertently) partly disinhibit the amygdala.
36 Since the amygdala is known to stimulate the PVN, amygdalar disinhibition might actually affect the PVN more than a lesion intended to target the projections from the medial PFC to the hypothalamus.
37 Furthermore, because the amygdala is also a site of CRF production,
38 an investigation evaluating the regions relevant to regulation of the HPA axis would ideally also evaluate activity of the amygdala. Thus, in order to assess intact neural networks, neuroendocrine lesion studies may be well complemented by functional neuroimaging paradigms.
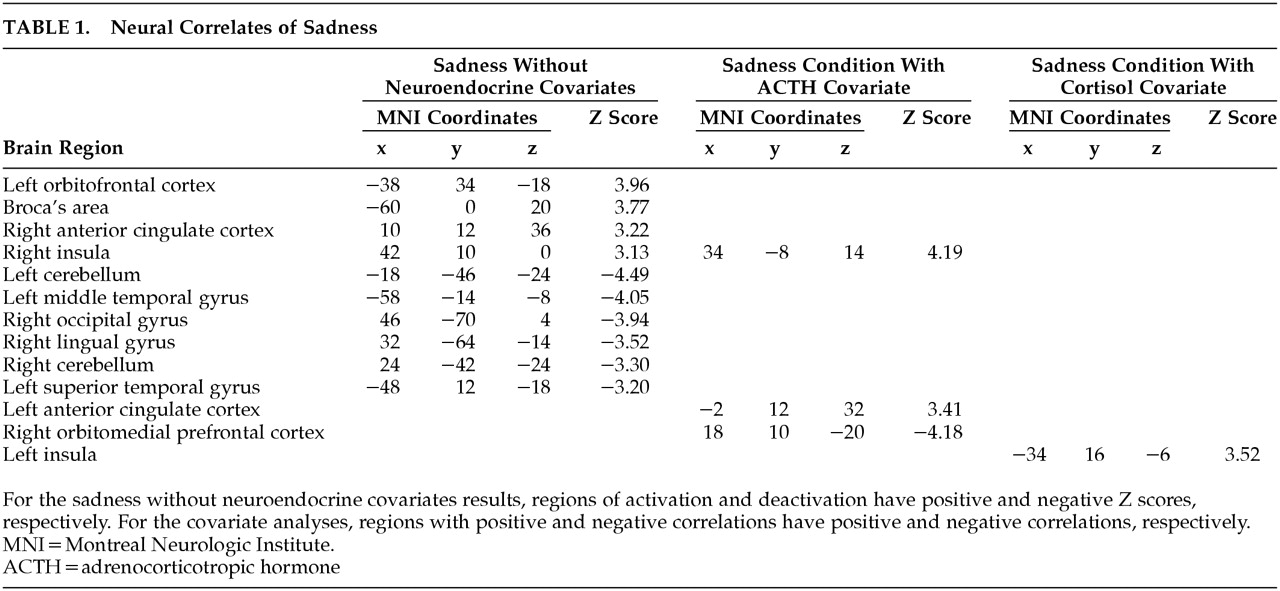
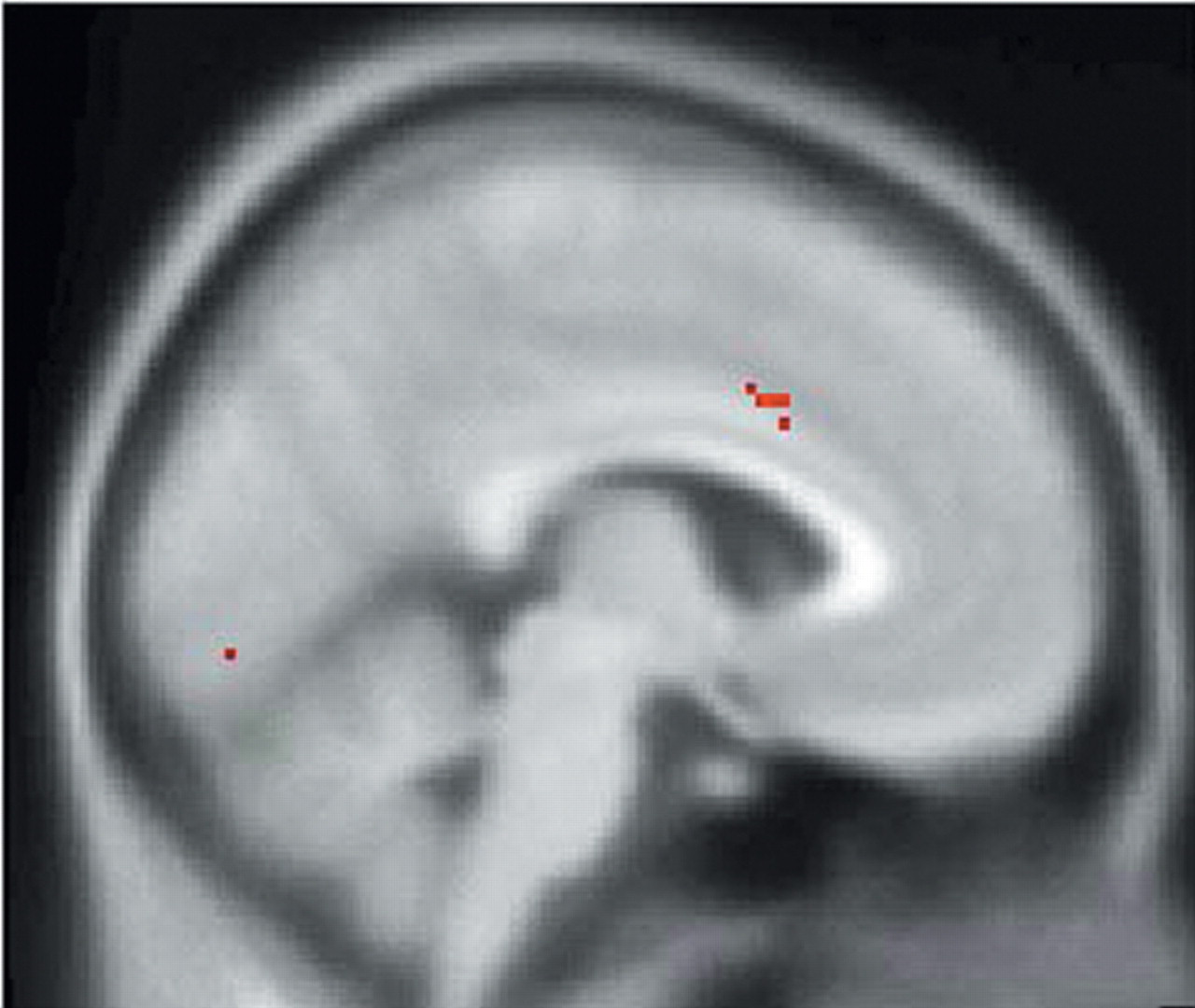
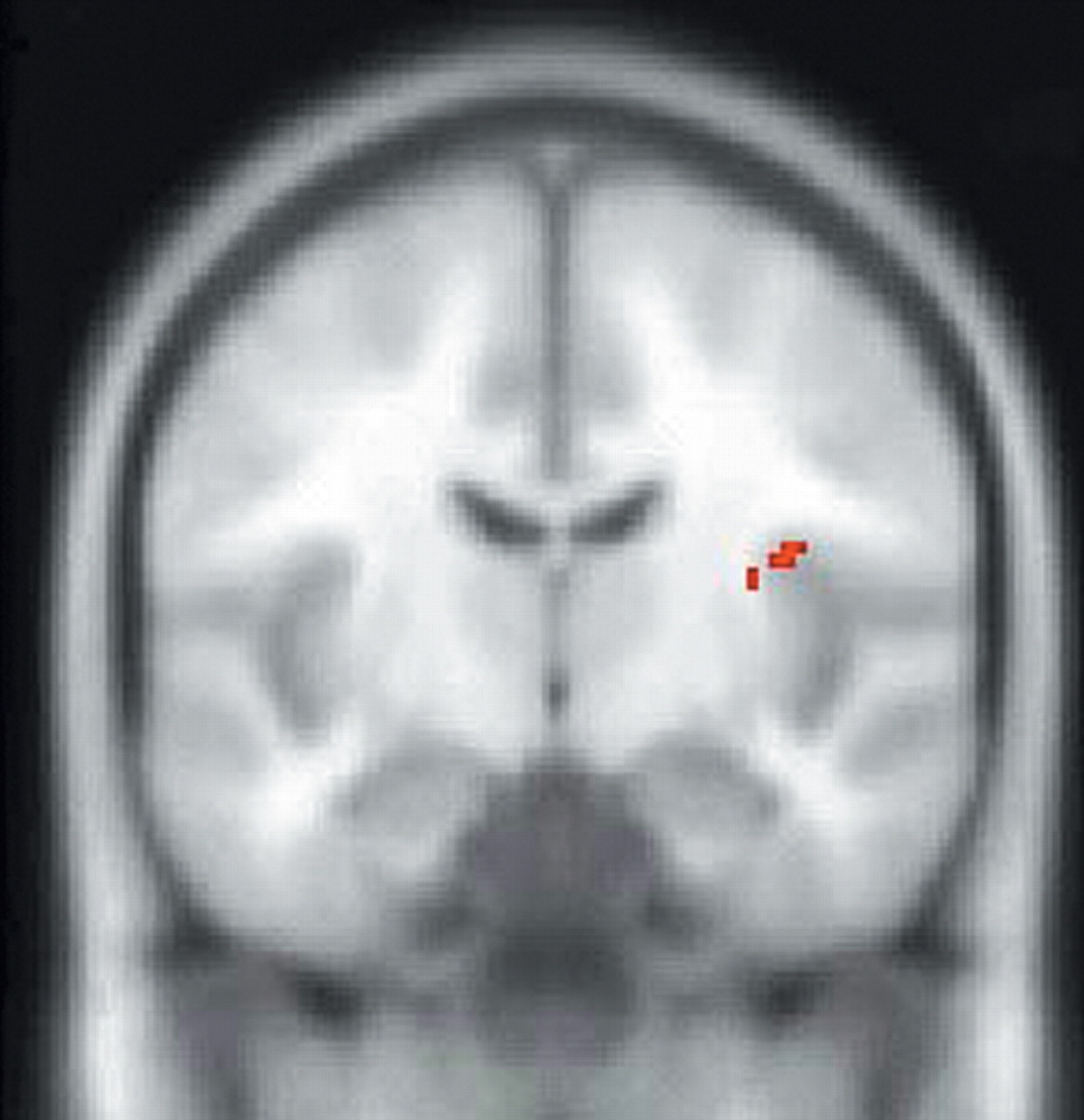
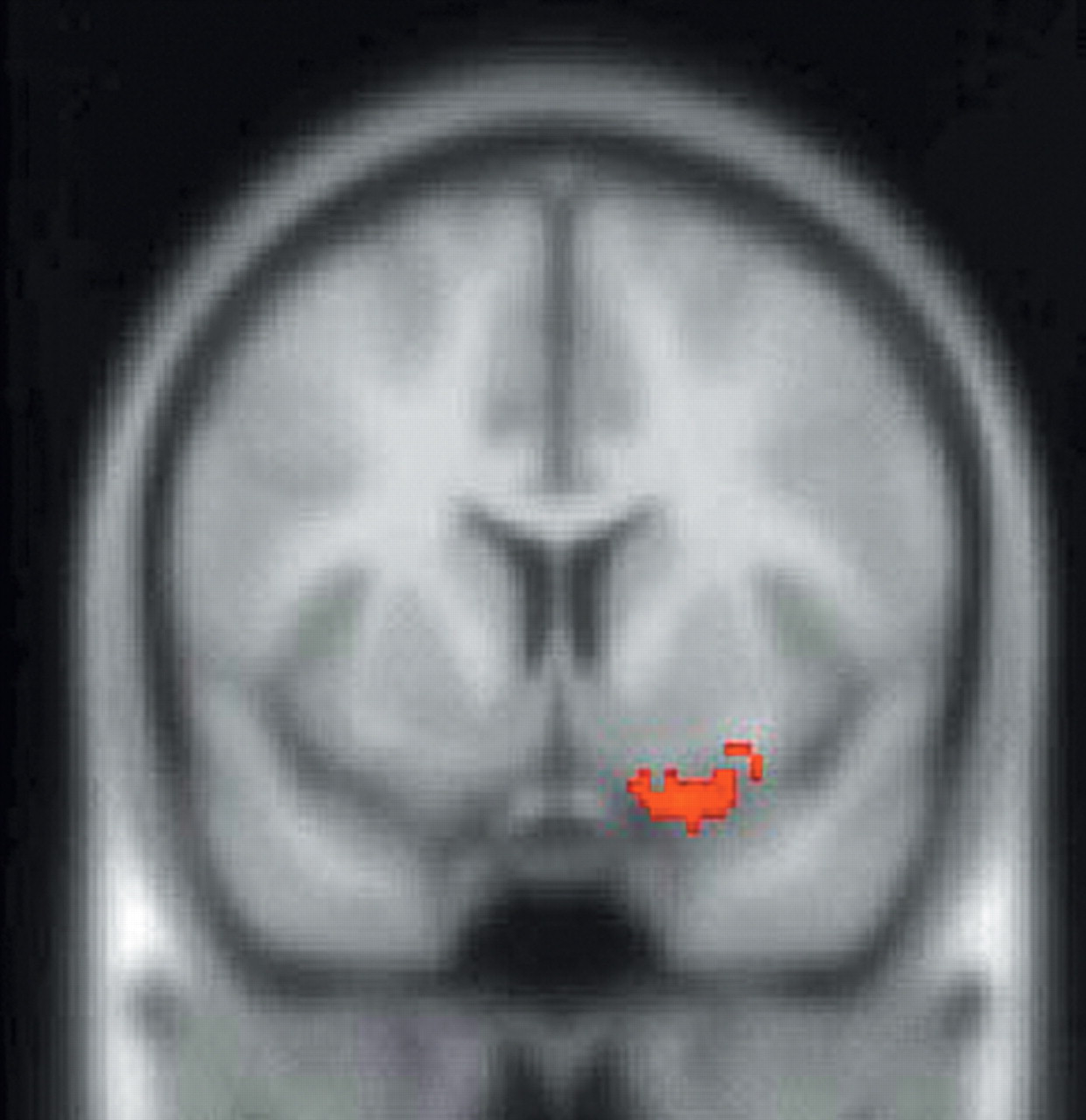
In support of the aforementioned animal studies, SPM endocrine covariate analysis in this study showed that ACTH measures were positively correlated with rCBF in the left anterior cingulate cortex and negatively correlated with rCBF in the right caudal OFC/ventromedial PFC, thus providing further evidence that the orbitomedial PFC and ACC are important regions involved in regulation of the HPA axis. In addition, ACTH measures were positively correlated with rCBF in the right insula. The complementary covariate analysis, which used peripheral cortisol levels as the covariate of interest, showed a positive correlation with rCBF in the left insula, a region that has projections to the amygdala and ACC and may, therefore, indirectly affect regulation of the HPA axis.
39Hence, our SPM covariate analysis further delineates components of the neural network regulating the HPA axis. These findings complement studies that have used sadness induction as an heuristic for investigation of the functional neuroanatomy of MDD.
14 Mayberg et al. evaluated the neuroanatomical relationship between sadness and depression through comparison of the neuroanatomical regions recruited during sadness in healthy volunteers to the neuroanatomical regions that showed metabolic change in patients recently recovered from a depressive episode and showed significant overlap between these two conditions.
13 As a logical extension of this heuristic since we have shown that the ventromedial prefrontal, anterior cingulate, and insular cortices may be related to regulation of the HPA axis during sadness, aberrations of these structures in depressed subjects may account for some of the suprahypothalamic dysregulation of the HPA axis observed in some subjects with MDD (in addition to the putative involvement of the amygdala and hippocampus).
40In this regard, the caudal OFC, ventromedial PFC, and anterior cingulate cortex are sites that have been repeatedly implicated in the pathophysiology of MDD. For example, a subregion of the ventromedial PFC, the subgenual PFC, has been shown to have a smaller volume in subjects with MDD when compared to healthy volunteers.
41 Furthermore, after correcting for reduced volume, the subgenual PFC shows increased rCBF in the active phase of MDD.
42–44 Because the caudal OFC and ventromedial PFC have direct projections to the amygdala and structures that regulate the PVN,
45,46 both of which are major sites of CRF production
10,38,47 metabolic aberrations of the caudal OFC/ventromedial PFC may indirectly contribute to the elevation in CRF
2 and the cortisol dysregulation
1 found in many depressed subjects.
Different methodological dimensions of our study also merit discussion. One limiting feature was our use of SPECT as opposed to positron emission tomography (PET). Position emission tomography offers greater resolution of deep structures such as the hippocampus and amygdala, which are structures of relevance to emotion and regulation of the HPA axis.
9,10 Single photon emission comuted tomography may not provide sufficient resolution to adequately evaluate these structures. Secondly, an issue of relevance to the endocrine dimension of our paradigm was the absence of a significant change in cortisol levels in response to sadness induction. The absence of this finding may not, however, have much practical significance. Our ACTH levels did increase, and it has been shown that not all ACTH pulses necessarily result in increases in cortisol levels.
35 Furthermore, this issue did not preclude our main goal, which was identification of the neuroanatomical regions involved in the
regulation of cortisol and ACTH during sadness. The presence of adequate variance in hormone levels across subjects (in response to sadness induction) allowed for the use of an SPM covariate analysis to identify the regions that uniquely correlated with this variance. Thirdly, the time course of sadness induced brain activity, and subsequent PVN, pituitary, and adrenal activity is difficult to evaluate. However, the typical delay of approximately 5–10 minutes between peripheral increases in ACTH and consequent adrenal release of cortisol
48 suggests that the timing of our collection of peripheral neuroendocrine measures adequately brackets the temporal window of radiotracer capture by the brain.
Our paradigm correlates central neural activity and peripheral endocrine activity through the collection of endocrine measures at time points closely bracketing the temporal window of radiotracer injection and thereby differs from psychoendocrine paradigms that assess cortisol levels at a time point(s) that may not correspond to the cerebral activity responsible for genesis of the plasma level. Thus our methodology is oriented toward a more direct functional evaluation of the HPA axis. As an extension of this paradigm, the dynamic nature of the HPA axis may be more fully evaluated by pairing serial PET scans (or possibly functional MRI) with more frequent collection of neuroendocrine measures (e.g., possibly every minute). Furthermore, follow-up studies must evaluate more patients: the sample size of eight subjects in this study (with only six subjects with adequate data for endocrine covariate analysis), was reasonable to merely investigate a new methodology. The small sample size of our study limits the generalizability of our findings and requires replication with a greater sample size. However, our demonstration of an exclusive correlation of neuroendocrine measures with the ventromedial prefrontal and anterior cingulate cortices, regions with strong theoretical backing
3,49 suggests that this methodology may be a robust and reasonably specific means by which to investigate the suprahypothalamic neural network that regulates the HPA axis.
In conclusion, our study has investigated both neural and endocrine dimensions of sadness in healthy, young adult women. Sadness (independent of endocrine activity) was associated with increased rCBF of the left OFC, right ACC, and right insula, findings that have also been observed in other studies.
15,33 Independent of our investigation of neural activity, we also evaluated the endocrine response to the mental state of sadness, which showed increase of ACTH levels in the sadness induction relative to the neutral induction but no statistically significant change in cortisol levels. Our primary goal, which was identification of the neuroanatomical regions that may be involved in the regulation of ACTH and cortisol during sadness, identified the orbitomedial prefrontal, anterior cingulate, and insular cortices as possible regions in the regulation of ACTH and the insula as a possible regulatory site for cortisol. These sites have been shown to be affected in MDD and may account for the increase in CRF and cortisol found in MDD. Finally, our methodology was intended to acknowledge the dynamic nature of the HPA axis and apply an omnibus evaluation to the investigation of intact networks regulating this axis.
ACKNOWLEDGMENTS
We thank R. Connors, V. Gayshan, and S. Genna of Innovative Medical Imaging, Waltham, MA; R. Zimmerman of the Department of Nuclear Medicine at Brigham and Women’s Hospital, Boston ; and S. Weise and A. Bonab of the Department of Nuclear Medicine at Massachusetts General Hospital, Boston, for their technical assistance.
Innovative Medical Imaging donated use of their SPECT camera and Nycomed-Amersham donated the radiotracer for this study. Additional support was provided by the Clinical Research Training Program, Department of Psychiatry, Harvard Medical School (W.E.O.), and NIMH grant K23 MH-01735 (D.D.D.).
Portions of this artice were presented at the 13th Annual American Neuropsychiatric Association meeting, March 9–12, 2002, La Jolla, California and the 12th Annual Rotman conference on Emotions and the Brain, March 25–26, 2002, Toronto, Ontario, Canada.