Cocaine abuse is a substantial public health concern, as recent estimates indicate that there are 1.5 million chronic cocaine users in the United States.
1 Societal costs of drug abuse including cocaine abuse are estimated to be $62 billion.
2 The use of cocaine produces changes in brain chemistry with attendant functional consequences. Neuroimaging studies have revealed metabolic
3,4 and structural
5 differences in prefrontal brain regions, including the anterior cingulate cortex (ACC) and lateral prefrontal cortex (LPFC) of cocaine and polydrug abusers relative to non-drug-using comparison subjects. The caudal-dorsal ACC has strong reciprocal interconnections with the LPFC, and this network operates during performance of tasks that involve high levels of mental effort.
6 This neural network also appears to participate in executive cognitive functions (ECF) governing resolution of conflict, response inhibition, performance monitoring, implementation of control, and error monitoring.
7–11 Disruption of these functions could impair an individual’s ability to monitor and inhibit inappropriate behavior. In the context of substance abuse, such disruption could impede the discontinuation of self-destructive, drug seeking behavior, thereby undermining treatment.
The aim of this investigation was to determine if cocaine abusers have impaired function of the ACC and LPFC. We used water method positron emission tomography (PET H
215O) imaging and a cognitive activation task, a modified version of the Stroop Task.
12–15 The Stroop is a task of cognitive control and performance monitoring that requires response inhibition and suppression of a more habitual response in favor of an atypical one.
10,16,17 Positron emission tomography (PET) and frontal magnetic resonance imaging (fMRI) studies in normal volunteers demonstrate that the ACC and LPFC have complementary functions underlying the performance of different aspects of the Stroop Task.
9,10,17,18 The LPFC plays a role in maintaining attentional demands of the Stroop Task whereas the ACC plays a role in conflict monitoring.
10 It is important to study the functional integrity of the ACC and LPFC in cocaine abusers because dysregulation of this prefrontal neural network might contribute to the development and persistence of maladaptive behaviors (e.g., poor response selection) that could sustain addiction or impede abstinence from drug use.
In previous neurobehavioral studies of abstinent cocaine abusers, we have shown a dose-related relationship between the amount of cocaine used and performance on tasks associated with ACC function (Stroop) and LPFC (CALCAP—sequence of numbers match-to-sample task).
19,20 Based on this work, we predicted that abstinent cocaine abusers would show impaired function of the ACC and LPFC, compared with a non-drug-using comparison group. We also reasoned that if abnormalities in brain functioning were directly related to cocaine use, changes in brain activation in these regions during performance on the task should be correlated with the amount of cocaine used.
Discussion
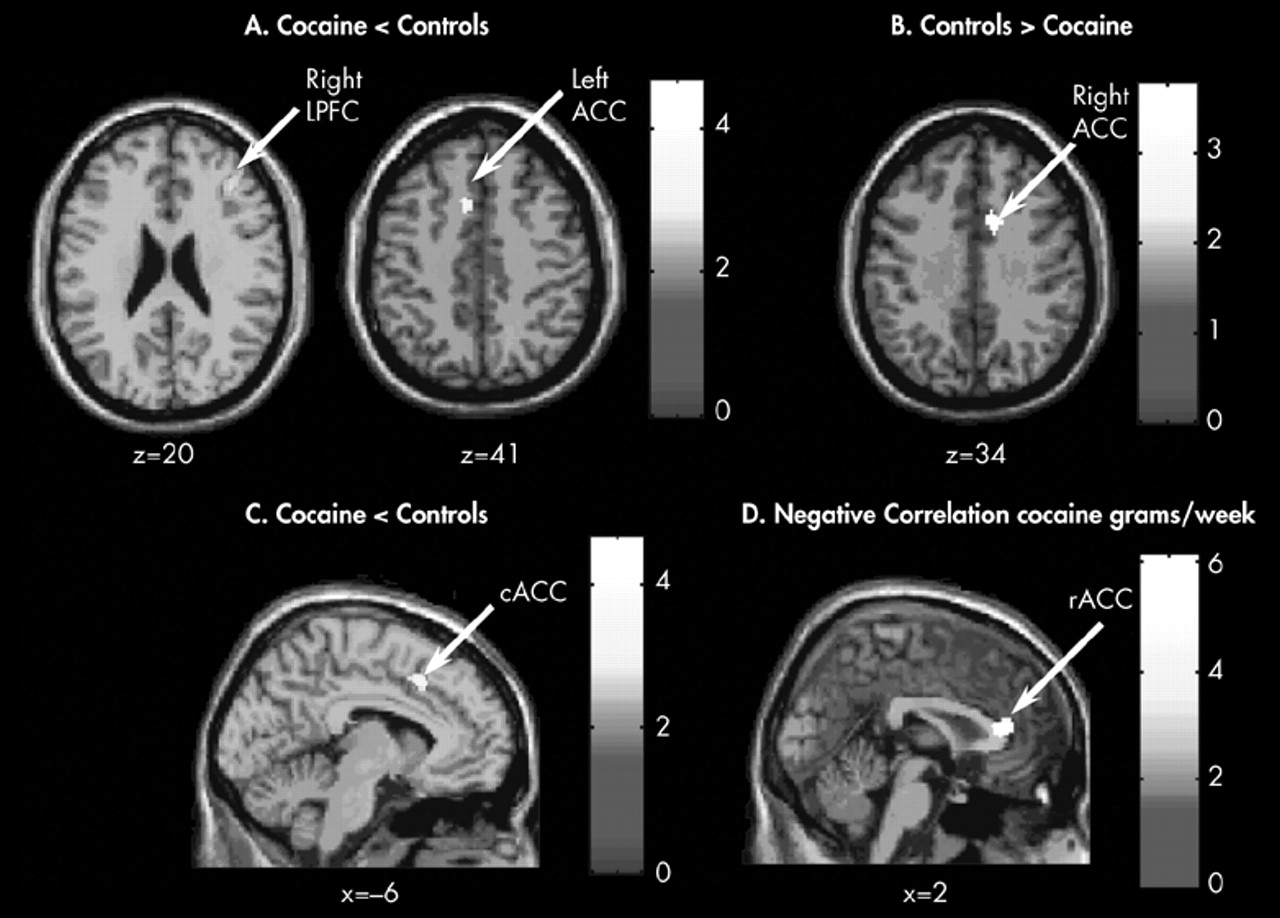
Abstinent cocaine abusers showed less activation than comparison subjects in the left ACC and right LPFC and more activation in the right ACC when performing a modified Stroop Task, suggesting a conflict-related effect. In addition, the amount of cocaine used prior to the 23-days of enforced abstinence was negatively correlated with large areas of activation in the rostral-ventral (infragenual) ACC and right LPFC. These findings suggest that 23-day abstinent cocaine abusers exhibit persistent functional brain abnormalities in prefrontal brain circuits involved in executive cognitive function (ECF) and that the effects are related to cocaine use since those with the highest cocaine use also showed the lowest activation in prefrontal regions. Compromised prefrontal brain circuitry, and thus difficulty with ECF may favor susceptibility to addiction or resistance to treatment.
Although we cannot determine causality from this study, the dose-related association between the amount of cocaine administered and activations in the rostral-ventral ACC and right LPFC supports the hypothesis that chronic, heavy use of cocaine contributes, at least partly, to persistent prefrontal cortical dysfunction. One possible mechanism for cocaine-induced prefrontal cortical dysfunction relates to the vasoconstrictive actions of cocaine and the purported associated ischemic changes.
32 However, we believe that reduced activation in the left ACC and right LPFC and increased activation in the right ACC in cocaine abusers is a specific effect rather than a general phenomenon since no between-group differences were found in brain activity in the resting scans, no conflict scans, or in the pattern of activation in the left motor cortex. Another possible mechanism for dysfunction of the ACC and LPFC may relate to tissue volume loss in these specific brain regions. In the identical sample of cocaine users and comparison participants that were described in this study, the cocaine group showed smaller brain volumes in the ACC and LPFC, as tested with voxel-based morphometry (VBM).
33 It may be impossible, however, to separate cerebrovascular from neuronal mechanisms to explain the alterations in brain activity in cocaine abusers.
Although the two groups studied differed in mean age and proportion of individuals who were smokers, we found no differences in activation between smokers and nonsmokers. Also, no significant correlations were observed between age and activation in the ACC or LPFC in our entire group of 26 participants.
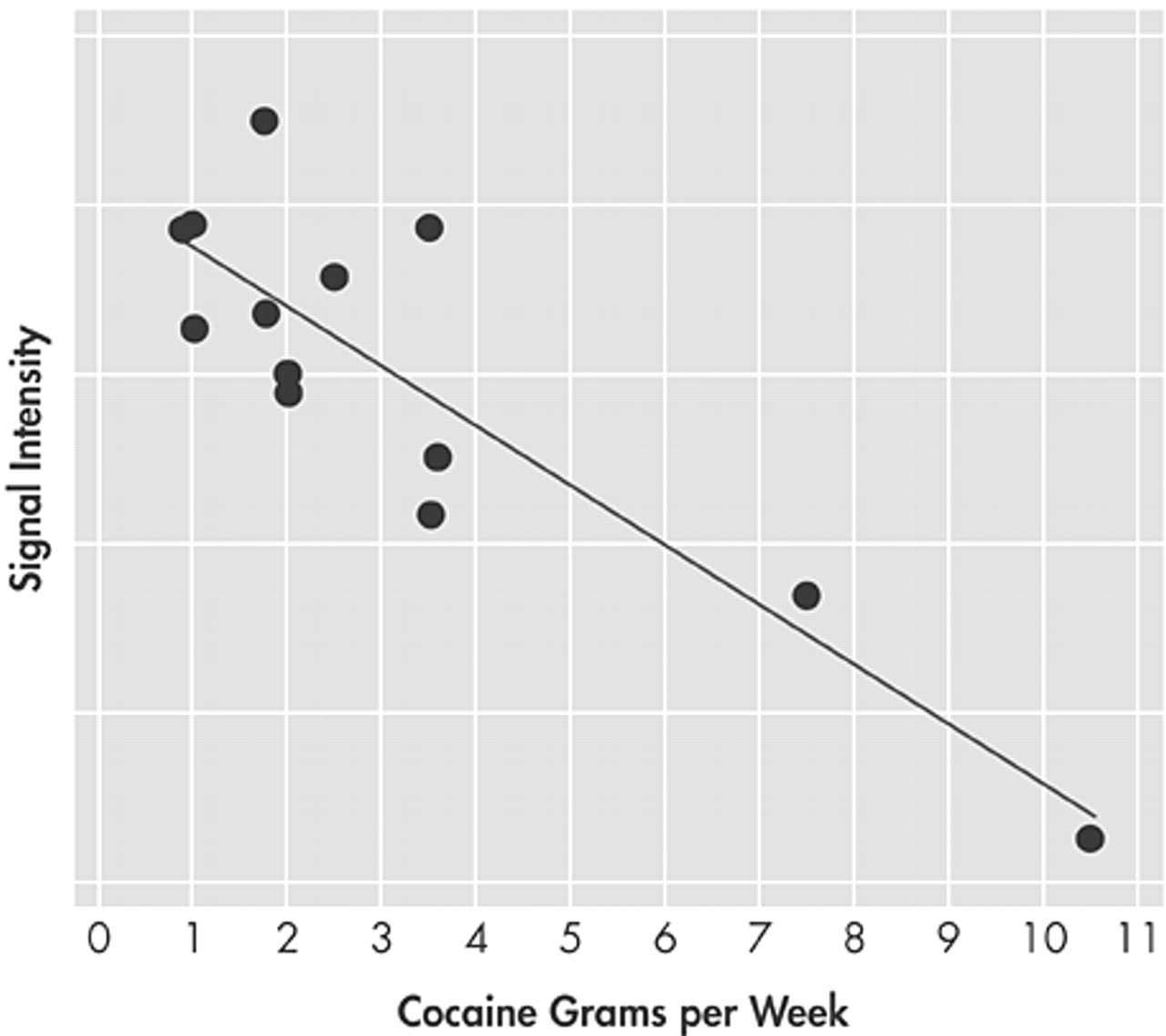
Grams of cocaine used were negatively correlated with activation in the rostral-ventral division of the ACC (infragenual) (
Figure 1D), whereas group differences in activation during task performance were found in divisions of the caudal-dorsal (midcingulate) ACC (
Figure 1C). The rostral-ventral division of the ACC is believed to play a role in emotional processing, whereas the caudal-dorsal division is involved in cognitive processing.
6,34 It therefore, appears that the amount of cocaine intake influenced function of the emotional—rostral—ventral division of the ACC. Blunting of activation in a brain region associated with emotion may have important ramifications for the development and persistence of drug dependence, especially since higher amounts of cocaine use were associated with lower activation in the emotional division of the ACC. More cocaine use was also associated with lower activation in the right LPFC. Similar negative associations between cocaine use and brain activity in these two brain regions likely reflect the reciprocal connectivity between these two regions.
9,15Cocaine abusers did not show poorer behavioral performance than non-drug-using comparison subjects on either condition of our modified Stroop Task. One explanation for this finding is that cocaine abusers may utilize different neural pathways than those used by the comparison subjects to perform our task. This hypothesis is supported by our finding of higher activation in the cocaine users in the right ACC, possibly reflecting a compensatory mechanism. Nevertheless, reports on the laterality of activation in the ACC during performance on other versions of the Stroop Task are mixed. Some studies report only right-sided activation. Other studies report left-sided activation, and some report bilateral activation.
35 In this study, we found both right and left ACC group differences in activation in the same caudal-dorsal division of the ACC as observed by others in normal subjects and schizophrenics.
11,14,15 Our neuroimaging findings are, therefore, biologically plausible and consistent with those of others. Further speculation about the significance of the differences in function between the right and left ACC would be premature since little has been described about the functional asymmetry of the ACC. The present data, however, suggest that an asymmetry exists.
Another possible reason for the lack of group differences in performance is the weaker sensitivity of behavioral assays compared with neuroimaging for detecting abnormalities or differences in brain functioning.
31 Indeed, on the standardized Stroop Task used for clinical purposes, lower performance was associated with increasing cocaine use in 56 cocaine abusers.
20 Thus, between-group performance differences may become apparent with a larger sample size.
Although our data does not show behavioral evidence of impairment in ECF, we do show abnormalities in the brain regions responsible for the complex mental process of disassociation between intact behavior/performance and abnormal metabolic functioning, which may occur because regions downstream and parallel to the affected brain areas might subsume the role of dysfunctional regions. Cortico-cortical connections and distributed connections in the various cortical brain regions
36 may make such a compensatory mechanism viable. During periods of stress, however, when it might be important to inhibit certain behaviors (e.g., drug self-administration), cocaine abusers might find it difficult to use compensatory neural mechanisms to overcome faulty self-monitoring, especially in situations involving conflict (e.g., attempting to refrain from drug use in the presence of drug-related cues). Finally, impairments in ECF may be a common denominator in the evolution of maladaptive behaviors, not only in substance abuse but also in other neuropsychiatric disorders such as posttraumatic stress disorder,
37 obsessive-compulsive disorders,
38 and attention-deficit disorder.
39 An understanding of the neural mechanisms involved in executive cognitive control, especially under conditions of conflict, may elucidate the neural substrates involved in a variety of neuropsychiatric disorders.
ACKNOWLEDGMENTS
This study was supported by NIH grants DA 11426 (KB), the JHBMC-GCRC (MO1 RR02719), and the DHH NIDA Intramural Research Program.
The authors thank the nurses and clinical staff at NIDA-IRP, the Brain Imaging Center, and the Bayview GCRC who contributed to this project. We especially thank Debra Hill, B.A. for computer and database support and Kathy Winters, Ph.D. for the programming of the modified Stroop Task.