A lthough memory impairments in schizophrenia patients have been often reported, the extent and pattern of impairment are yet to be clearly defined.
1 Substantial evidence now exists showing neuropsychological dysfunction in schizophrenia patients,
2 but there are limited data available for determining whether the impairment is specific to schizophrenia.
3 Schizophrenia is often characterized as including compromised memory function, especially related to tasks that are sensitive to frontal
1 and temporo-hippocampal functions.
4 –
8 Schizophrenia patients often show difficulties with delayed recall of names, sentences, and other memory tasks, which is consistent with a lesion in the temporal lobe. They also show impaired immediate memory, which is found when there is a dysfunction in the frontal lobe.
9Schizophrenia studies using brain lateralization to examine memory impairments demonstrate that schizophrenia patients have a smaller left temporal lobe volume than healthy comparison subjects,
10 which suggests damage to the left rather than the right temporal lobe.
11,
12 However, some analysis, abstraction, and memory task studies suggest that there is dysfunction in both the left and the right hemispheres.
13 Using the assumption that the subtle interictal memory disturbance associated with idiopathic temporal lobe epilepsy is similar to the memory deficits in schizophrenia,
5,
14 Seidman et al.
15 compared the memory functions of schizophrenia and temporal lobe epilepsy patients, including separate considerations of cued recall, free recall, and saving score. Interpretations from the study,
15 however, are limited by the small sample of only 30 temporal lobe epilepsy cases and the fact that there was no differentiation between left temporal lobe epilepsy and right temporal lobe epilepsy. Furthermore, we only used Digit Span as an auditory attention task and did not include a task involving visual attention, which is known to be impaired in schizophrenia patients.
In a study by Gold et al.,
5 only five indices of memory derived from the Wechsler Memory Scale–Revised (WMS-R)
16 were used to compare the memory function. In another study by Gold et al.,
17 a thorough analysis of verbal memory functioning was carried out using the California Verbal Learning Test (CVLT).
18 Unfortunately, the rate of verbal memory retention could not be derived from only the saving score in these studies,
5,
17 because only short-term verbal memory was tested.
The purpose of our study was to investigate compromised memory function of the patients with schizophrenia. We hypothesized that schizophrenia patients would show poor immediate and delayed recall performances in verbal and visual memory tasks. We analyzed three patient groups (schizophrenia, left temporal lobe epilepsy, and right temporal lobe epilepsy) and compared their memory dysfunctions through memory tests that probed three aspects of memory: immediate recall, delayed recall, and retention.
Following the approach of Seidman et al.,
15 we divided verbal and visual memory tasks into immediate and delayed memory tasks from which retention rates were derived. Our study differs from that of Seidman et al.
15 in that we compared the performances of schizophrenia patients on verbal and visual memory tasks with those of left temporal lobe epilepsy and right temporal lobe epilepsy patients who have memory impairments that result from a clear lesion. We observed material-specific memory impairment in the present study, where the left temporal lobe epilepsy group showed severe verbal memory impairment while the right temporal lobe epilepsy group showed severe visual memory impairment,
19,
20 and we expected that schizophrenia group would show poor performances in both memory tasks.
Moreover, we considered that comprehensive neuropsychological tests cannot be administered to most schizophrenia patients because they lack the necessary attention.
15,
21 We thus included visual memory span
16 for the assessment of visual attention and analyzed a larger number of individuals. Both verbal and visual memory tasks were subdivided into easy (i.e., cued recall) and difficult (i.e., free recall) tasks for within-group comparisons.
METHOD
Subjects
A total of 241 subjects, including 76 with schizophrenia (40 men, 36 women) and 165 with temporal lobe epilepsy (88 men, 77 women), were analyzed by random sampling. All schizophrenia subjects were inpatients with no mental retardation (full-scale IQ [FSIQ] > 80) meeting the Diagnostic Statistical Manual of Mental Disorder fourth edition criteria.
22 Two psychiatrists made a consensus research diagnosis following reviews of patient records, extensive clinical observations, and repeated interviews.
All schizophrenia patients were diagnosed as the paranoid subtype. Potential subjects were excluded if they had a history of drug use, alcohol dependence, or an identifiable neurological disorder. We also excluded patients with clear extrapyramidal symptoms (EPS) with a score on Simpson-Angus Scale
23 > 0.9, and with tardive dyskinesia (score lower than 3 on Abnormal Involuntary Movement Scale, [AIMS]), and we excluded patients with prior electroconvulsive therapy (ECT). All patients with schizophrenia were taking fixed doses of either typical (haloperidol or chlorpromazine) or atypical (clozapine or risperidone) antipsychotic medication at the time of the study.
Epilepsy subjects comprised 165 patients with no mental retardation (FSIQ > 80) over 20 years of age with medication-resistant epilepsy of unilateral temporal lobe origin. None of the patients had any history of neurosurgical treatment. We collected the results of interviews, patient medical records, and medical and demographic information, which included neurologists’ diagnoses and neuroradiological reports for all cases of confirmed organic brain disorder. The epilepsy sample was composed of 93 left temporal lobe epilepsy patients and 72 right temporal lobe epilepsy patients. All patients had their epileptogenic foci identified by a combination of procedures, including 24-hour scalp electroencephalographic telemetry monitoring, magnetic resonance imaging (MRI), and neuropsychological testing. We excluded patients that did not have a temporal lobe focus. All patients in this study were tested as a part of their initial clinical research assessment while receiving a stable regimen of medication. All patients with medically intractable epilepsy were taking two or more antiepileptic drugs, after fixing maximal tolerable doses or maximal therapeutic doses, and were taking fixed doses at testing. Conventional antiepileptic drugs including carbamazepine (800–1200 mg/day), valproic acid (1000–2000 mg/day), and phenytoin (200–400 mg/day) were most commonly used. New antiepileptic drugs such as topiramate (100–200 mg/day), lamotrigine (200–400 mg/day), and oxcarbazepine (1200–1800 mg/day) were also used. For some patients, phenobarbital (90–150 mg/day) was used.
The healthy comparison group consisted of 30 subjects who lacked a history of psychological disorders, brain damage, or any psychological or neurological treatment, and all were living a normal, healthy life. Although we did not have controls for any specific variable, we aimed to obtain age- and economic-background-matched comparison subjects. Any subjects with a family history of psychosis or psychiatric hospitalization were excluded from the comparison group.
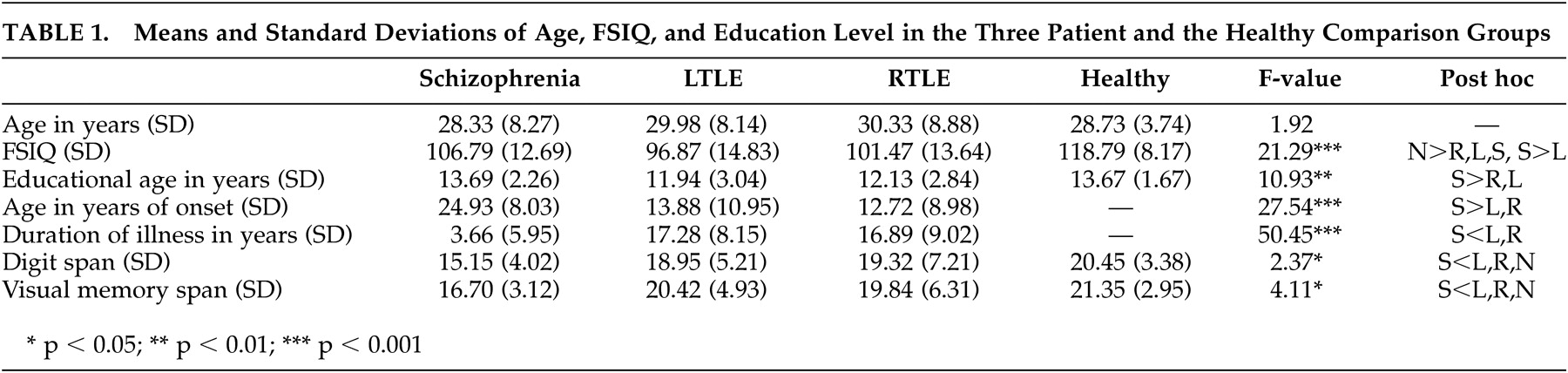
The clinical and demographic variables of the three patient groups and the healthy comparison group are presented in
Table 1 . The three patient groups did not differ in age. All subjects were right-handed and were screened for their history of head injury, alcohol abuse, and serious medical illness.
Neuropsychological Assessment
Because our goal was to minimize state-dependent effects, the patients were tested when they were judged to be clinically stable by the clinical staff familiar with them. A majority of patients (85.5%) were tested as outpatients. The data were analyzed using the methods below.
Korean Wechsler Intelligence Scale
24 : The FSIQ based on the standardized version of the Wechsler Intelligence Scale was used for this study.
Wechsler Memory Scale (WMS)
25 and WMS-R
16 : The WMS is a rapid, simple, and practical test,
25 but includes only one subtest that measures memory for visual material and lacks measures of long-term retention of learned material.
16The Logical Memory I (LM I) task consists of two brief stories that are related to the examinee. After each one, the examinee retells the story from memory. The Logical Memory II (LM II) task is a delayed-recall trial of LM I, administered 30 minutes after the first presentation of the stories. In the Verbal Paired Associates I (VEPA I) task, the examinee learns eight word pairs. VEPA II is a delayed recall trial of VEPA I. The Visual Reproduction I (VR I) task requires the examinee to draw from memory simple geometric designs that are each viewed for 10 sec. VR II is a delayed recall trial of VR I. Immediate recall task was added because it is absent from the WMS-R. VR I was used as an immediate recall task in this study, although it is usually used as a Visual Copy task. The Visual Paired Associates I (VIPA I) task requires the examinee to learn the color associated with each of six abstract line drawings. The Visual Paired Associates II (VIPA II) task is a delayed-recall trial of VIPA I.
The Verbal Paired Associates (VEPA) I and II tasks were used as easy and cued recall verbal memory tasks, and the Logical Memory (LM) I and II tasks were used as difficult and free recall verbal memory tasks. Subjects also completed VIPA I and II as easy and cued recall visual memory tasks and VR I and II as difficult and free recall visual memory tasks. Both verbal and visual memory were assessed for immediate and delayed recall. Auditory and visual attention were assessed by digit span and visual memory span tests. All neuropsychological tests were administered by two clinical neuropsychologists.
Data Analysis
Basic demographic and clinical variables were examined by one-way analysis of variance (ANOVA), and post-hoc analyses were conducted using the Scheffé-test. Our primary analysis incorporated intelligence and education as covariates in analysis of covariance (ANCOVA), since both of these factors are related to some aspects of memory performance and because the groups showed significant differences on these variables. To determine if there were significant group differences on memory performance over and above IQ or education, we also performed ANCOVA with these variables.
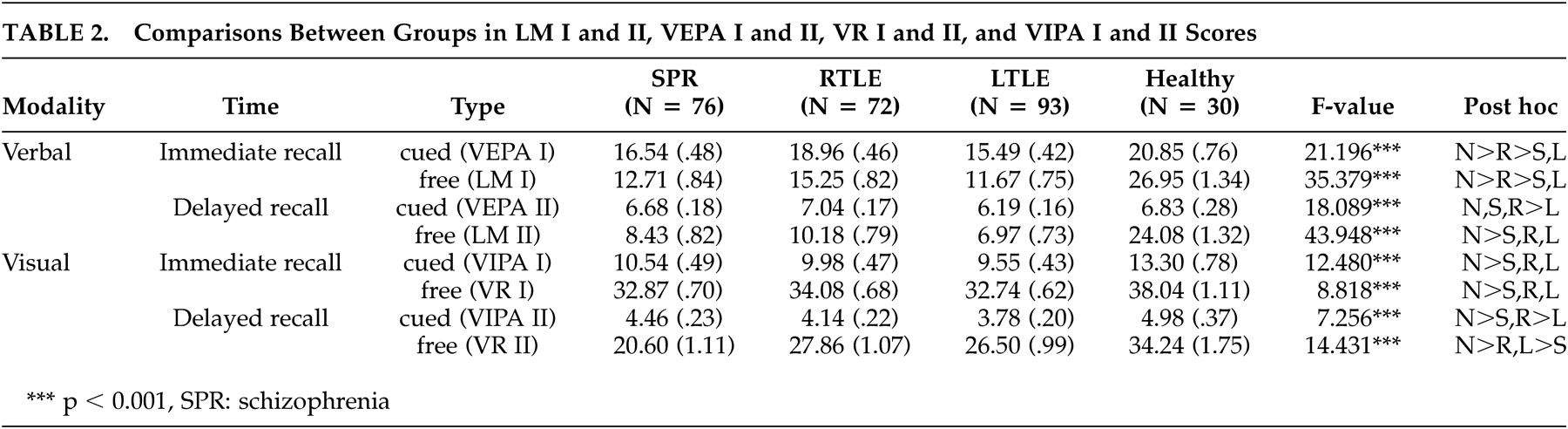
As shown in
Table 2, healthy comparison subjects achieved much higher scores than the three patient groups in all verbal and visual tasks. In this study, the number of healthy comparison subjects was only 30, and we wanted to know only the mean scores of the memory tasks. Thus we subsequently excluded the healthy subjects from further analyses of verbal and visual tasks.
Using LM I and II for verbal memory and VR I and II for visual memory, multivariate analysis of variance (MANOVA) was conducted to compare between-group differences (the three patient groups) and within-group differences (immediate and delayed memory processes) in verbal and visual memory.
The pattern of performance based on modality (verbal, visual) in the three patient groups was assessed by a mixed-model MANOVA, with diagnosis as the between-group factor and modality as the within-group factor. Similarly, the pattern of performance based on task (cued and free recall) in the three patient groups was assessed by a mixed-model MANOVA, with diagnosis as the between group factor and task as the within group factor.
Modality was created by averaging standardized test scores within each verbal and visual memory test. Task was created by averaging standardized test scores within each cued and delayed recall test. Standardization of the individual test scores to a mean of zero and an SD of one was performed using the control mean and the pooled standardization score within the three patient groups involved in the study. The retention rates in verbal and visual memory tests were derived according to the following calculations: verbal retention rate = (LM II/LM I) x 100; visual retention rate = (VR II/VR I) x 100. The difference in saving scores of the three patient groups was examined, and the Scheffé-test was conducted for post-hoc analysis.
Probability values less than 0.05 were considered statistically significant. Statistical analyses were carried out using (SPSS) version 10.0 (Chicago, U.S.).
RESULTS
Demographic and Clinical Variables
ANOVA showed that there were significant differences among the three patient groups and the healthy comparison group in intelligence (F=21.29, p<0.001) and education (F=10.93, p<0.001), but no significant difference in age. The three patient groups had lower FSIQ (intelligence) scores than the healthy comparison group. The left temporal lobe epilepsy patient group had the lowest FSIQ scores, while right temporal lobe epilepsy and schizophrenia patient groups did not differ significantly. The education levels of the healthy comparison group and schizophrenia patient group were significantly higher than both epilepsy groups, and the schizophrenia patient group had the shortest duration of illness among the three patient groups. The left temporal lobe epilepsy and right temporal lobe epilepsy groups did not differ significantly in education level or duration of illness.
Comparisons of Immediate and Delayed Recall in Easy and Difficult Tasks
Verbal Memory
Using ANCOVA with intelligence and education as covariates, the left temporal lobe epilepsy group showed the lowest scores for both immediate and delayed recall. On the easy immediate memory (cued recall) task (VEPA I), the schizophrenia group scored lower than the right temporal lobe epilepsy or the healthy comparison group, but they scored higher than the left temporal lobe epilepsy group. On the difficult immediate recall task (LM I), all three patient groups performed more poorly than the healthy comparison group, with the left temporal lobe epilepsy group showing a significantly impaired performance compared to the right temporal lobe epilepsy group. On both the easy (VEPA II) and difficult (LM II) delayed recall tasks, the schizophrenia and left temporal lobe epilepsy groups had the lowest scores (
Table 2 ).
Visual Memory
Using ANCOVA with intelligence and education as covariates, the three patient groups had significantly lower scores than the healthy comparison group in both immediate and delayed memory tasks. Post-hoc analyses showed no significant difference among the three patient groups for the immediate recall task. However, the left temporal lobe epilepsy group had the lowest score on the easy paired-association task (VIPA II), a measure of delayed recall, whereas the schizophrenia group had the lowest score on the difficult free recall task (VR II) (
Table 2 ).
Results of MANOVA
Verbal Memory
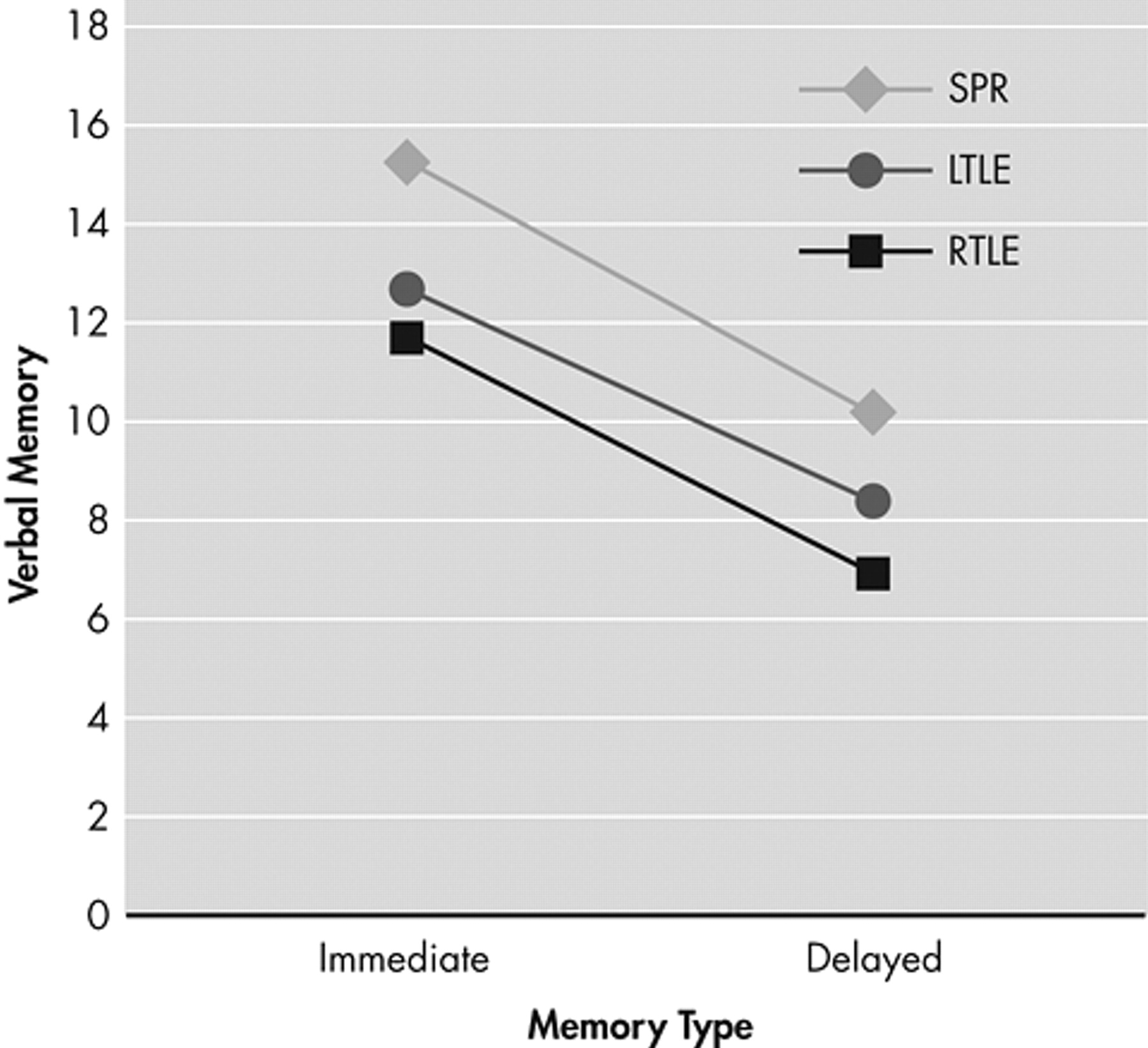
MANOVA showed no significant within-group (immediate, delayed) difference in verbal memory (F=0.01, p>0.97). The intergroup main effect (F=0.83, p>0.48) and intergroup and intragroup interaction (F=1.98, p>0.14) were also nonsignificant. The performance of the left temporal lobe epilepsy patient group was the poorest, followed by the schizophrenia and the right temporal lobe epilepsy groups, although these differences were not significant (
Figure 1 ).
Visual Memory
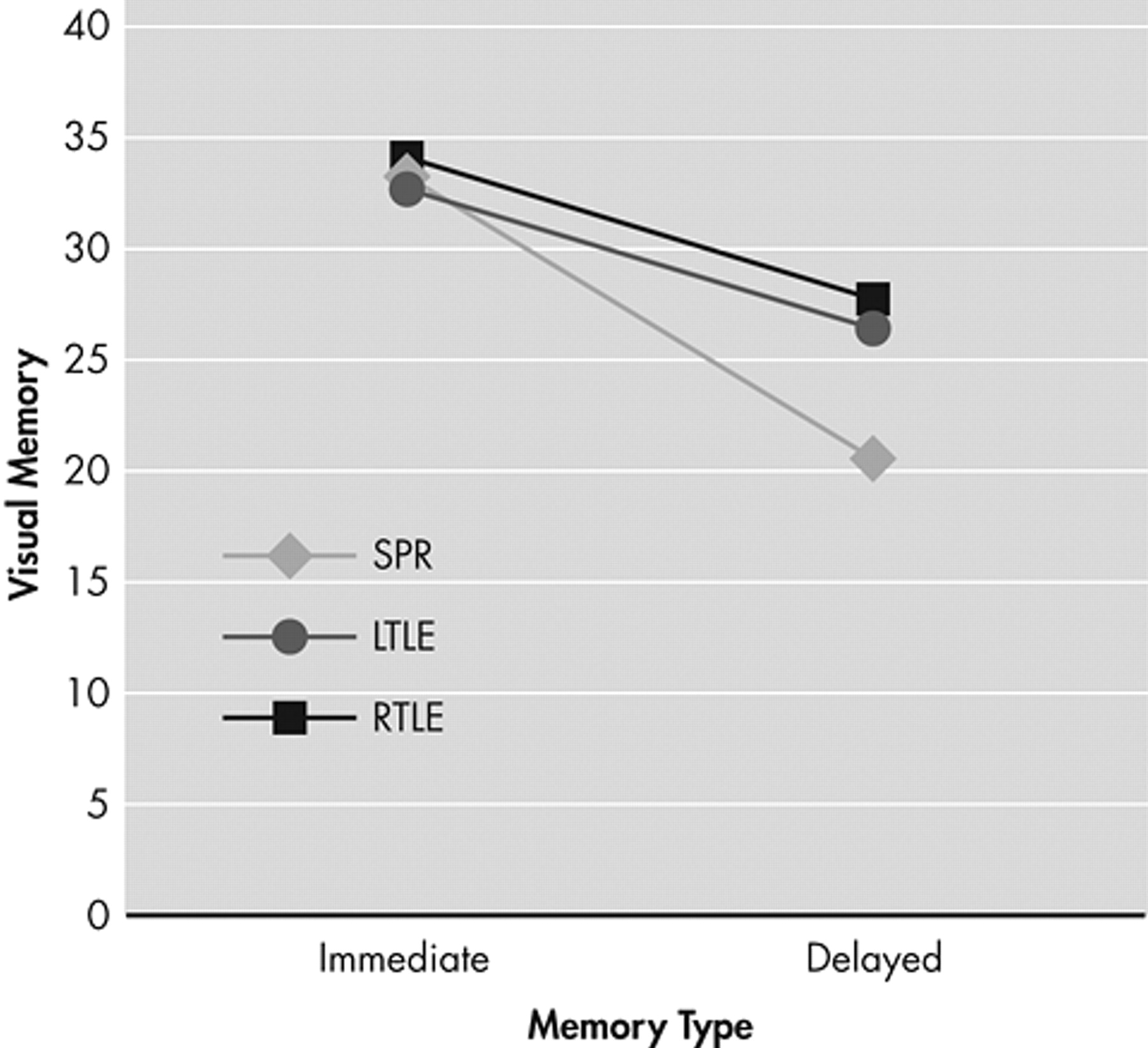
In terms of visual memory, a significant intragroup (immediate, delayed) main effect (F=9.76, p<0.01) was found owing to a significantly poorer performance in delayed recall than in immediate recall. An intergroup main effect (F=7.65, p<0.01) was also found, with the schizophrenia patient group showing a significantly more severe deficit in performance than the two epilepsy patient groups. However, no significant difference was found between the left temporal lobe epilepsy and right temporal lobe epilepsy groups. Finally, significant intergroup and intragroup (immediate, delayed) interactions (F=10.85, p<0.001) were found, demonstrating that the schizophrenia group had significantly lower performances on delayed recall than the right temporal lobe epilepsy group (
Figure 2 ).
A MANOVA with diagnosis (schizophrenia, left temporal lobe epilepsy, and right temporal lobe epilepsy) as the between-group factor and modality as the within-group factor revealed no significant difference by modality (verbal, visual) interaction (F=1.14, p=0.46), suggesting that the three patient groups showed similar patterns. A MANOVA with diagnosis (schizophrenia, left temporal lobe epilepsy, and right temporal lobe epilepsy) as the between-group factor, and modality as the within-group factor revealed no significant difference by task (easy, difficult) interaction (F=0.89, p=0.39), suggesting similar patterns (but not levels) in the three groups.
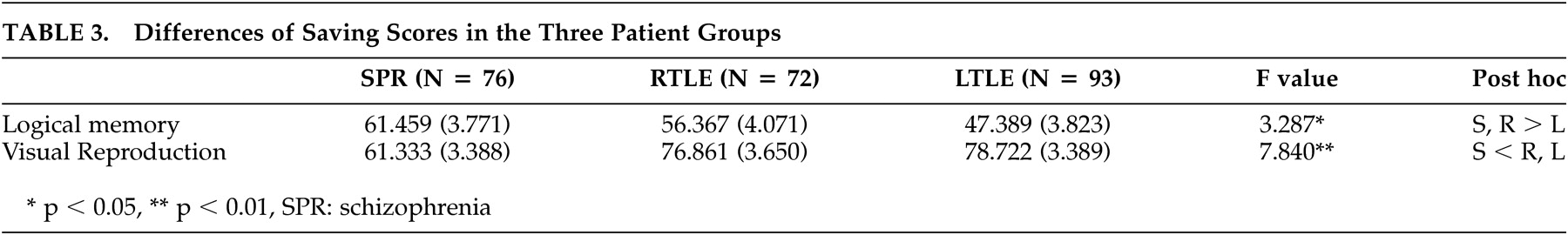
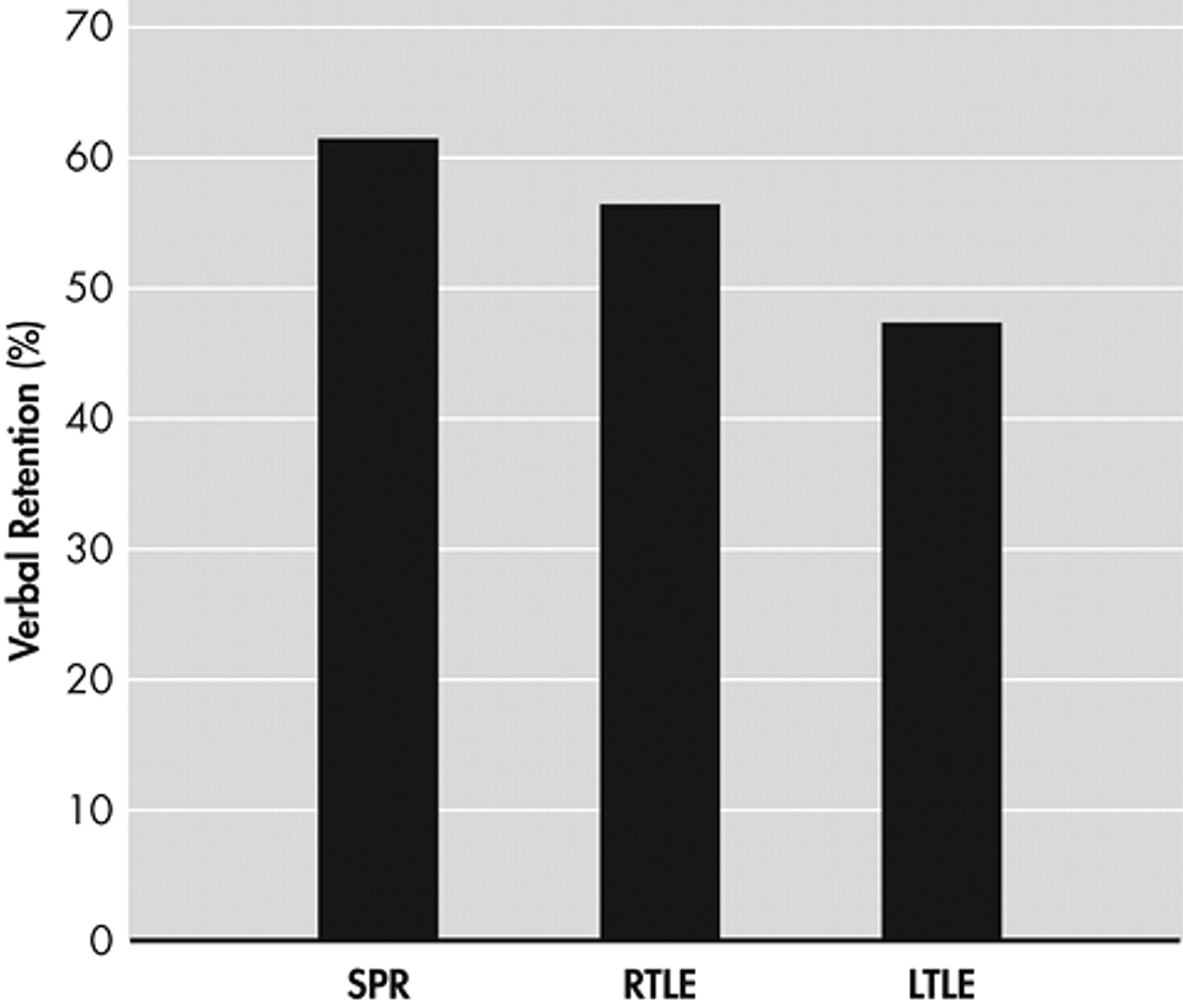
Comparison of Retention Rates of the Three Patient Groups
A significant difference was found in the retention rates of verbal tasks among the three patient groups (
Table 3 ). While the left temporal lobe epilepsy group showed the poorest performance, the schizophrenia and right temporal lobe epilepsy groups showed relatively high retention rates with no significant difference between the two groups (
Figure 3 ). In the visual recall task, the schizophrenia group showed significantly poorer performance compared to the two epilepsy groups, between which performances did not differ significantly (
Figure 4 ).
DISCUSSION
The results of this study show a moderate impairment in immediate and delayed verbal memory in the schizophrenia patients, and the impairment of visual memory was amplified with delayed recall and retention. The hypothesis of this study that schizophrenia patients would show poorer immediate and delayed recall performances in verbal and visual memory tasks was confirmed.
We found that left temporal lobe epilepsy patients had the lowest FSIQ scores and that the schizophrenia group had a higher educational level than the left temporal lobe epilepsy and right temporal lobe epilepsy groups. The relatively lower levels of education observed in the left temporal lobe epilepsy and right temporal lobe epilepsy groups may be attributed to an earlier age of onset and longer duration of illness than the schizophrenia group. Such long-term cognitive impairments could have made attaining further education more difficult.
26The three patient groups showed significantly lower performances on verbal memory tasks than the healthy comparison group. Although the schizophrenia patients had an unimpaired retention rate, they found free recall (LM I) more difficult than cued recall (VEPA I). This suggests that, unlike in temporal lobe epilepsy patients, the reduction of immediate memory processing is closely related to working memory in schizophrenia patients.
27,
28 At the same time, the schizophrenia group also demonstrated a poorer performance in the delayed memory task, which was similar to the pattern observed in patients with temporal lobe lesions.
The verbal memory of the patients in the left temporal lobe epilepsy group showed severely impaired levels of immediate and delayed recall in both easy (VEPA I and II) and difficult (LM I and II) tasks. The retention rate derived from the saving score was also the lowest in the left temporal lobe epilepsy group. These results support the idea that left temporal lobe epilepsy patients have a material-specific verbal memory impairment
19,
20,
29 and that left-sided lesions are associated with verbal memory impairment and episodic memory recall deficits.
30,
31 The poor performance of the left temporal lobe epilepsy patients may result from their weakness in relational memory because the verbal task used in this study was a logical memory task to retell a story
16 that was designed to assess their memory of prose.
32In visual memory tasks, all three patient groups performed poorly, compared to the healthy subjects. In terms of immediate recall, all three patient groups showed lower scores than the healthy subjects both in cued recall (VIPA I) and free recall (VR I), but there was no significant difference among the three patient groups. In delayed recall, the right temporal lobe epilepsy group scored lower than the healthy comparison group, which is inconsistent with material-specific memory impairment.
19,
20,
33 No significant difference was found between the right temporal lobe epilepsy and the left temporal lobe epilepsy groups in delayed recall.
Although the right temporal lobe epilepsy group showed poorer performance in free recall than the healthy comparison group, they outperformed the schizophrenia group. We also found that the left temporal lobe epilepsy group performed poorly even in the cued recall task. Schizophrenia patients, however, showed relatively less impairment when given cues, but they had the largest deficit among the patient groups when no cues were given. In addition, they received significantly lower saving scores than both epilepsy groups in the visual memory score, which was a composite of the VR I and II scores.
In a study by Bell et al.,
34 left temporal lobe epilepsy and right temporal lobe epilepsy patients and healthy subjects were assessed on auditory and visual selective reminding tests (SRTs). The right temporal lobe epilepsy group performed worse than the healthy subjects on trial 6 from the auditory SRT and on all three variables from the visual SRT. These results have similarities to the findings of the present study. However, while the two temporal lobe epilepsy groups in the Bell et al. study did not differ from each other on any of the SRT variables, the left temporal lobe epilepsy group in the present study showed the poorest performance among the three patient groups in visual delayed and cued recall. It was an unexpected result that the schizophrenia group showed the lowest performance in the visual delayed and free recall task in our study because it is usually expected that right temporal lobe epilepsy patients would show lower performances in visual tasks than either of the two other patient groups.
These results highlight the importance of carefully considering the task material before interpreting the performances of schizophrenia patients in memory tasks. For example, because the subjects can use verbal mediation (i.e., verbally memorizing what the figure looks like in their mind) when performing VIPA I and II as well as VR I and II tasks, their performance may not reflect any lesion in the brain structure or function responsible for visual memory.
35 Our results suggest that memory tasks used in a clinical setting may lack sensitivity for assessing right temporal lobe epilepsy patients.
15,
29Although WMS-R
16 and the Rey Complex Figure Test (RCFT)
36 often have been used as visual recall tasks in memory studies, they have been reported to contain more object recall than location recall elements.
31 Based on the findings of this study, we suggest that it would be better to choose sensitive visual memory tests that contain a location recall element, such as the family pictures subtest contained in WMS-III, for future studies of memory function in schizophrenia.
37Cognitive deficits in schizophrenia patients are enduring features per se and cannot be considered as secondary to psychiatric symptoms or to the adverse effects of medication.
38 Nevertheless, our study is limited in that we did not assess motivation variables of the patients and did not control for the duration of illness of patients, nor their medication regimes, given that both the epilepsy and schizophrenia patients in this study had been medicated individually based on their medical conditions.
CONCLUSIONS
We observed a moderate impairment in immediate and delayed verbal memory in schizophrenia patients, and the impairment of visual memory was amplified with delayed recall. Such a result can be interpreted not only as a generalized cognitive deficit, but also as an integrative dysfunction involving the mesial temporal and frontal lobes in the left and right hemispheres, whereby the lesion site cannot be determined selectively.
9,
32 Our results show that selection of a memory task that cannot be influenced by verbal mediation is very important for analyzing memory dysfunction in schizophrenia patients.