Patients with schizophrenia have increased rates of several chronic medical conditions, including coronary artery disease (
1,
2,
3 ), chronic obstructive pulmonary disease (
4 ), HIV (
5,
6,
7 ), hepatitis C (
7 ), and diabetes mellitus (
8,
9 ). The management of chronic medical illness requires that patients accomplish a series of tasks, including engaging in activities that promote health, monitoring physical symptoms and signs, and interacting with health care providers (
10 ). It has been proposed that greater levels of psychiatric symptoms might lead to greater medical comorbidity because poor attention and poor insight might create an inability to self-monitor and follow medical regimens (
11,
12,
13 ).
Few studies have specifically addressed the question of whether patients with schizophrenia who have more severe symptoms of mental illness also have more severe medical illness or whether medical comorbidity results in disability over and above that which results from schizophrenia. In a longitudinal study of 124 institutionalized geriatric patients with schizophrenia, neither the number of medical diagnoses at baseline nor an increased number of medical diagnoses over time contributed to a change in functional status (activities of daily living) over the four-year study period (
14 ). A study of 719 patients with schizophrenia from the Schizophrenia Patient Outcomes Research Team suggested that the presence of more severe psychiatric symptoms is associated with more severe medical comorbidity and poorer physical health status (
15 ). In that study, medical comorbidity was measured by patient self-report of the presence of 12 medical conditions, physical health status was assessed with a single item from the Lehman Quality of Life Interview (
16 ), and psychiatric symptoms were assessed with the depression and psychoticism subscales of the 90-item Symptom Checklist (
17 ). Although medical comorbidity was not associated with greater severity of psychotic symptoms in bivariate analyses, multivariate analyses revealed a significant association between number of current medical conditions and psychoticism, after the analyses adjusted for age. This study did not specifically evaluate functional status.
Comorbidity refers to the total burden of illnesses across multiple potential conditions unrelated to the patient's principal diagnosis (
18 ). There is no widely accepted measure for medical comorbidity, but the number of diagnoses has been widely used as a measure of comorbidity (
19 ) and has been shown to predict mortality (
20 ). A recent large prospective primary care study compared the ability of five commonly used measures of medical comorbidity to predict mortality and health care costs over one year. In this study, all the comorbidity measures significantly predicted increasing total costs (charges), an increased number of ambulatory visits, and an increased probability of an inpatient admission or death. Diagnosis-based measures, including a simple count of comorbid conditions, had greater predictive validity for one-year mortality than medication-based measures (
21 ).
The study presented here aimed to evaluate the interrelationship of psychiatric symptoms (depression, neurocognitive functioning, and positive and negative symptoms of schizophrenia), medical comorbidity, and psychosocial functioning in a sample of patients with schizophrenia. The study utilized the baseline data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) trial. The CATIE study is a multisite trial of antipsychotic pharmacotherapy funded by the National Institute of Mental Health, which collected baseline data from more than 1,400 patients with schizophrenia at more than 50 sites in the United States between 2001 and 2003 (
22 ). In this study, baseline data from the CATIE study were used to examine two questions. First, is increased severity of psychiatric symptoms among patients with schizophrenia associated with greater medical comorbidity? Second, what is the association of medical comorbidity with functional outcomes among patients with schizophrenia, independent of the contributions from psychiatric symptoms and demographic characteristics?
Methods
Sample
The CATIE study was designed to compare the effectiveness of currently available first- and second-generation antipsychotic medications through a randomized trial. Participants were 1,493 men and women aged 18 to 65 years who met
DSM-IV criteria for schizophrenia. However, data from one site (33 patients) were excluded because of concerns about their quality. Participants gave written informed consent to participate in protocols approved by local institutional review boards. Details of the study design and entry criteria have been presented elsewhere (
22 ). A wide spectrum of patients with schizophrenia was enrolled in the trial in order to make the results as generalizable and representative as possible. There were few exclusion criteria, and patients with medical or psychiatric comorbidity or those who required concomitant other medications were included. Patients were excluded, however, if they had a myocardial infarction in the previous six months, history of or current QTc prolongation, uncompensated congestive heart failure, sustained cardiac arrhythmia, first-degree heart block, or complete left bundle branch block (
22 ). The study presented here utilized baseline data collected before randomization and the initiation of experimental treatments.
Measures
The diagnosis of schizophrenia was confirmed by structured psychiatric interview, the Structured Clinical Interview for DSM-IV (SCID) (
23 ). Baseline data included questions about age, race, gender, marital and educational status, and source of income, including earned income, Social Security payments (Supplemental Security Income or Social Security Disability Insurance [SSDI]), or compensation and pension payments from the Department of Veterans Affairs.
Medical comorbidity. In this study, two measures of medical comorbidity were evaluated: a simple count of medical diagnoses and physical health status, as measured by the score on the physical component summary subscale of the 12-item questionnaire from the Medical Outcomes Study (SF-12 PCS). Medical disorders and physical symptoms reported by participants during the baseline interview were categorized into medical diagnoses according to the Medical Dictionary for Regulatory Activities (MedDRA). MedDRA is an international medical terminology designed to support the classification, retrieval, presentation, and communication of medical information throughout the regulatory cycle of pharmaceutical products and biomedical devices (
24 ). The variable used for analyses was a simple count of the MedDRA diagnoses.
The second measure of physical health status was the SF-12, a validated instrument with normative data from clinical samples and the general population (
25 ). The SF-12 generates a score on the PCS that ranges from 0 to 100, with higher scores reflecting better health status. These scores have been standardized for the general population, such that a score of 50 reflects an average level of physical health, and each 10-point change in score reflects one standard deviation (
25 ). The SF-12 PCS was included as a secondary measure of medical comorbidity, as it is not based on specific medical diagnoses, and it is not subject to the risk of underreporting of medical diagnoses by patients.
Schizophrenia symptoms. Schizophrenia symptom severity was assessed with the rater-administered Positive and Negative Syndrome Scale (PANSS), which yields a total symptom score, based on 30 items that are rated from 1 to 7 (higher scores indicate more severe psychopathology), as well as subscale scores that reflect positive and negative symptoms (
26 ).
Depression symptoms. Depression was measured with the Calgary Depression Rating Sale, a nine-item clinician-administered scale designed to assess severity of depressive symptoms among patients with schizophrenia, as it does not contain items that overlap with negative symptoms of schizophrenia (
27 ). Items are scored on a scale of 0 to 3, with higher scores indicating more severe depression; mean scores were reported.
Alcohol and drug use. Co-occurring alcohol and drug use was assessed by the Alcohol Use and Drug Use Scales, which are widely used and accepted clinician rating scales for this population (
28 ).
Neurocognitive assessment. Neurocognitive functioning was measured by a neurocognitive assessment battery, which includes 11 measures of verbal fluency, working memory, verbal learning and memory, social cognition, motor function, attention, and executive function. Scores on these tests were converted to z scores and combined to construct five separate scales (processing speed, verbal memory, vigilance, reasoning, and working memory), which were then averaged to form a composite neurocognitive functioning score (
29 ).
Medication side effects. The CATIE schizophrenia trial utilized several measures of side effects, which occur commonly with antipsychotic medications, including the Barnes scale for akathisia (possible scores range from 0 to 5) (
30 ), the Abnormal Involuntary Movement Scale for tardive dyskinesia (possible scores range from 0 to 42) (
31 ), and the Simpson-Angus scale for extrapyramidal symptoms (possible scores range from 0 to 4) (
32 ). Higher scores reflect more severe movement abnormalities.
Psychosocial functioning. Psychosocial functioning was assessed by using the Heinrichs-Carpenter Quality of Life Scale (
33 ), a clinician-rated scale of psychosocial functioning, which has been validated and widely used in clinical trials of treatment of schizophrenia. The scale contains 21 items. Possible scores range from 0 to 6, with higher scores reflecting better functioning. Three of the four subscales were evaluated in this study: instrumental roles, interpersonal relations, and intrapsychic foundations. The number of days employed in the previous month was also used as a measure of functional status.
Analytic methods
First, bivariate associations were examined between each of the two measures of comorbidity (the count of medical conditions and the score on the SF-12 PCS) and demographic characteristics (age, gender, race, educational level, and marital status) and clinical characteristics (schizophrenia symptoms, depression symptoms, neurocognitive functioning, and extrapyramidal side effects).
Multivariate regression models were used to determine the additional impact of medical comorbidity on five measures of functional status (days employed and the total score and the three subscale scores of the Heinrichs-Carpenter Quality of Life scale). These simple linear regression models were conducted in three stages, with the first stage involving significant covariates (p<.05) from the bivariate analyses. The second stage involved the addition of the medical comorbidity variable (either the count of medical conditions or the SF-12 PCS), evaluation of the significance of this term in the regression models, and calculation of the change in the variance explained by the model (change in R 2 ) with the addition of this covariate. Separate regression models were conducted for each of the two medical comorbidity measures. The third stage evaluated whether the impact of medical comorbidity on functioning was greater in the presence of more severe schizophrenia symptoms. This analysis involved the addition of terms for interactions between medical severity and schizophrenia symptoms into the regression models.
Results
Altogether, 1,460 participants completed the baseline interview for the CATIE study. A total of 1,424 (97.5 percent) were not missing any data, and analyses were conducted on this sample. Participants with no missing data were similar to the sample as a whole except that they were more likely to be male, had significantly lower mean PANSS scores (positive, negative, and total), and were significantly more likely to be receiving public support or SSDI.
Table 1 presents the demographic and clinical characteristics of the sample, which had a mean age of 40.6 and was 73 percent male and 35 percent African American. On average, patients had mean PANSS scores of 75.5, somewhat lower than samples in other clinical trials of pharmacotherapy for schizophrenia, which have had mean PANSS total scores of 87 to 92 (
34,
35 ). The lower scores in the CATIE trial reflect the goal to enroll a more representative sample of patients with a wide range of schizophrenia severity. Participants in our study sample had, on average, mild to no depressive symptoms and a mean of 2.2 medical conditions. Mean physical functioning scores on the SF-12 PCS were 48.6, close to the normative mean of 50 for the general population (
25 ).
Overall, 58 percent of participants had at least one medical condition, and 9 percent of the sample had four or more medical conditions (
Table 1 ). The most common chronic medical conditions were hypertension (20 percent), hyperlipidemia (14 percent), and diabetes mellitus (11 percent), consistent with previous reports of samples of persons with schizophrenia (
36 ).
The correlates of the two measures of medical comorbidity in the sample are presented in
Table 2 . Bivariate correlation analyses revealed highly significant moderate associations between the count of medical conditions and the demographic characteristics of age (older patients had a greater burden of medical illness) and gender (women had a significantly greater number of medical conditions). The correlations between an increased number of medical conditions and greater depressive symptoms, increased neurocognitive impairment, and all three measures of antipsychotic medication side effects (akathisia, tardive dyskinesia, and extrapyramidal symptoms) were also statistically significant, but these effects were of a much smaller magnitude (
Table 2 ). An increased number of medical conditions was not associated with an increased likelihood of alcohol or drug abuse. There were similar significant bivariate associations between the SF-12 PCS and demographic characteristics, depression, and neurocognitive impairment (
Table 2 ).
As shown in
Table 2, a greater number of medical conditions was not associated with an increase in either the total PANSS score or the score on the positive or negative symptom subscale on bivariate analyses. Worse physical functioning (lower SF-12 PCS score), however, was statistically significantly associated with an increase in the total PANSS score and positive symptoms (but not negative symptoms). A greater number of medical conditions was not associated with any of the measures of functioning in bivariate analyses. There were, however, significant bivariate associations between the SF-12 PCS score and the number of days worked in the past 30 days and all the scales on the Quality of Life instrument (
Table 2 ), but these effects were quite small (
ρ =.1).
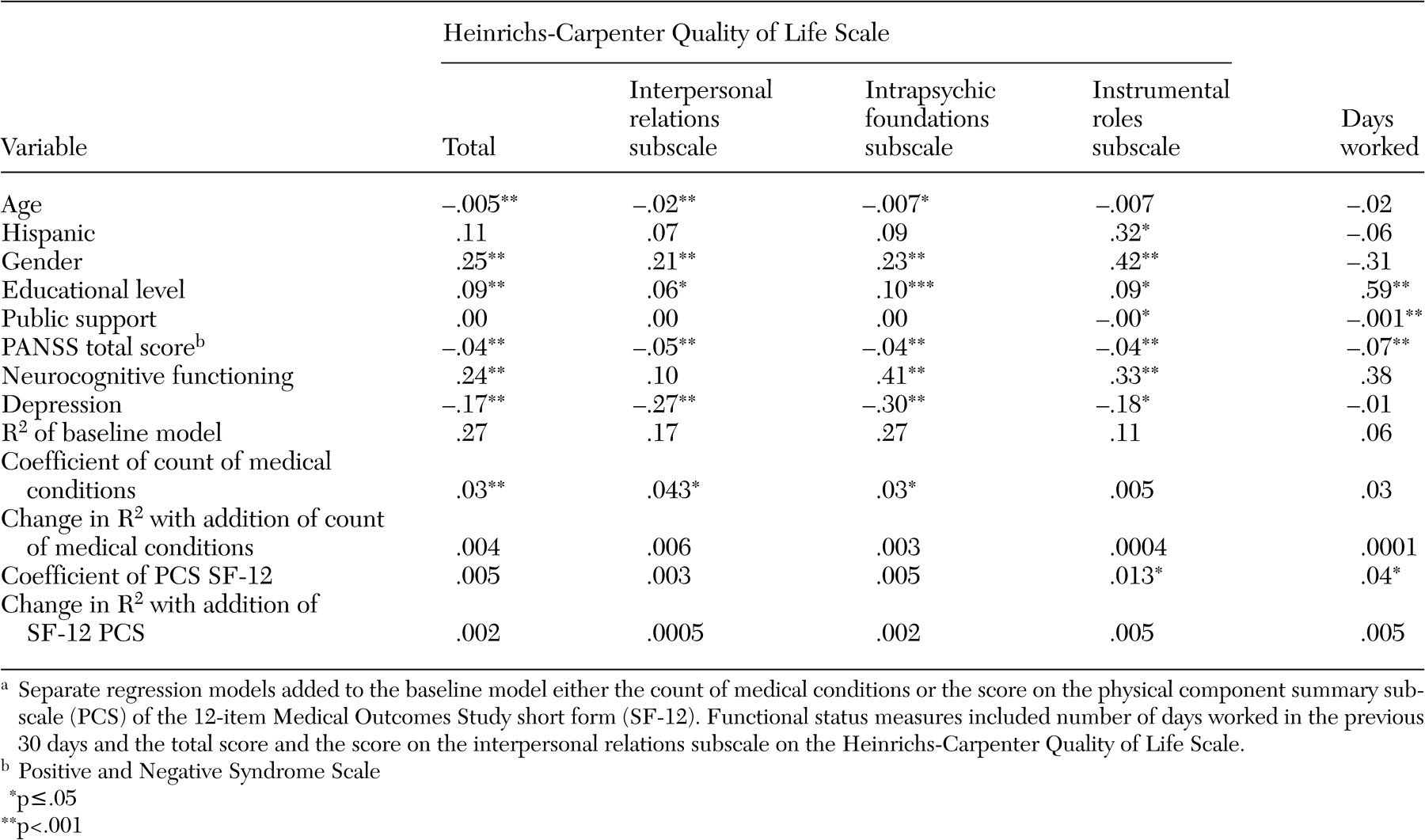
Separate multivariate linear regression analyses were conducted in order to determine the impact of medical comorbidity on the following measures of functional status: the number of days worked in the past 30 days and psychosocial functioning as measured by the total score and subscale scores of the Heinrichs-Carpenter Quality of Life Scale (instrumental roles, interpersonal relations, and intrapsychic foundations subscales). The results of these analyses are summarized in
Table 3 ; higher scores on the Heinrichs-Carpenter instrument represent better functional status. Demographic characteristics and psychiatric measures explained 6 to 27 percent of the variance in these functional status outcomes. The count of medical conditions contributed significantly to the models that predicted the total score on the Quality of Life Scale and the interpersonal and intrapsychic subscales but not to the models predicting the score on the instrumental subscale or the number of days worked in the past 30 days (
Table 3 ). Despite highly statistically significant associations, however, the magnitude of the effects were quite small (increasing the R
2 by .4 percent).
The SF-12 PCS was significantly associated with the number of days worked in the past 30 days and with the score on the instrumental roles subscale of the Quality of Life Scale (but not the total score or the score on the other subscales). Like the count of medical conditions, the SF-12 PCS explained only a very small percentage of the variance of either of these functional outcomes (
Table 3 ). When interactions between schizophrenia symptoms (total PANSS score) and either the count of medical conditions or the score on the SF-12 PCS were added to regression models of the functional outcomes, none of the interaction terms was statistically significant.
Discussion
In this large sample of patients with schizophrenia, a greater number of medical conditions was associated with greater severity of depression and neurocognitive impairment, even after the analyses controlled for severity of schizophrenia symptoms. There were, however, no significant associations between the count of medical conditions and more severe symptoms of schizophrenia, either positive or negative symptoms. Increased medical complexity was associated with tardive dyskinesia and akathisia, but the association with extrapyramidal symptoms was not significant after the severity of schizophrenia symptoms was controlled for.
The findings of this study are consistent with the extensive body of literature describing the associations between depression and chronic medical illness in primary care samples (
37,
38,
39 ) and suggest that these relationships also exist for patients with schizophrenia. The relationship between medical illness and neurocognition, however, is poorly understood among patients with schizophrenia (
14 ). Medical illnesses such as diabetes (
40 ), hypertension (
41 ), and peripheral arterial disease (
42 ) have been found to be associated with poorer neurocognitive function in nonpsychiatric populations. Many of these medical illnesses and their treatments have effects on the central nervous system that may be responsible for worsening of cognitive function. It is possible that this relationship is exacerbated among patients with schizophrenia for a variety of reasons. Because neurocognitive function among patients with schizophrenia is so severely impaired, with most patients falling in the lowest 10 to 15 percent of the population in the most important domains of cognitive function (
43,
44 ), persons with schizophrenia may be particularly challenged in their ability to access, engage with, and adhere to sufficient medical care (
45 ), particularly as they age (
46 ). Thus, not only may medical illnesses cause additional cognitive impairment, but cognitive impairment may also lead to the onset and exacerbation of these medical comorbidities among patients with schizophrenia.
The two measures of medical comorbidity evaluated in this study, the count of medical conditions and the score on the SF-12 PCS, were weaker correlates of several measures of psychosocial and occupational functional status than either severity of depression symptoms or neurocognitive impairment. Even in more conservative bivariate analyses, the score on the SF-12 PCS had highly significant associations with all measures of psychosocial functioning, but the magnitude of these effects was quite small. These findings are consistent with previous findings from a longitudinal sample of geriatric patients with schizophrenia (
14 ), which suggested that, as burdensome as medical comorbidity is for patients with schizophrenia, it is a weaker determinant of functional status than depression symptoms or neurocognitive impairment.
There are several possible explanations for the weak association between the count of medical conditions (or physical health status) and psychosocial and occupational functioning. It is striking that although patients in this study had close to normal physical functioning, as evidenced by the average SF-12 PCS scores of 48, they did have quite poor social and instrumental functioning (for example, only 27 percent had any type of employment). It is therefore possible that the lack of association between medical comorbidity and functional status was due to a "floor effect," such that among patients with severe disability resulting from psychiatric illness, physical illness cannot make social functioning much worse.
Alternatively, it is possible that despite significant psychiatric and social impairment, the patients enrolled in the CATIE trial were managing their chronic medical conditions quite effectively, which might be suggested by the mean physical functioning score that was close to the population norm. The finding of a SF-12 PCS score close to the population norm is consistent with previous studies of patients with serious mental illness, including studies that were specifically focused on improving treatment of patients with chronic medical conditions (
36,
47 ).
Some potential limitations of the study should be recognized. First, it is possible that patients in this study had less burden of medical illness than typical patients with schizophrenia. Potential participants were excluded from this study if they were too medically unstable to tolerate a clinical trial (myocardial infarction in the previous six months or uncompensated congestive heart failure). Other medical exclusions included cardiac arrhythmias that put patients at increased risk from treatment with antipsychotic medications (prolonged QTc interval, sustained arrhythmia, atrioventricular block, and left bundle branch block). But CATIE, unlike other clinical trials for treatment of schizophrenia, specifically aimed to include patients with a wide range of comorbid medical conditions. Furthermore, the rates of several chronic medical conditions in this sample were similar to those reported in other studies of medical illness among people with serious mental illness (
36 ).
Second, the measurement of medical comorbidity in this study may have been imprecise. Several recent studies have shown that information provided by patients about medical diagnoses is reliable, valid, and useful (
48,
49,
50,
51 ). But the self-report of medical diagnoses may still underrepresent a patient's true burden of illness, as patients who have limited access to medical care may have chronic medical conditions that are undiagnosed (
52 ). In this study, the self-report of diagnoses and symptoms was not confirmed by medical record review or consultation with medical providers. Interestingly, the SF-12 PCS, a measure of physical health status, did have significant bivariate associations with the total PANSS score and the positive symptom subscale score. This might suggest that persons with schizophrenia have undiagnosed medical conditions (perhaps reflecting decreased access to medical care), that they are unaware of their medical diagnoses, or that they are more reliable in reporting symptoms than diagnoses. Finally, because this study is cross-sectional and relied exclusively on baseline data, it may have failed to capture important longitudinal associations between medical comorbidity and the development of functional impairment among patients with schizophrenia. Further analysis of longitudinal data would illuminate this possibility.
Conclusions
The findings of this study have important clinical implications. Among patients with schizophrenia, depression and neurocognitive impairment were associated with an increased burden of comorbid medical illness and had stronger association with impaired functioning than the number of medical illnesses per se. Treatment of chronic medical conditions in this population might best be achieved by integrating medical illness management into a broader psychosocial treatment plan that comprehensively addresses all these complex comorbid conditions. It may be particularly important to evaluate impairment in physical functioning, regardless of medical diagnosis.
Acknowledgments
This article is based on results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project, supported by grant NO1-MH-90001 from the National Institute of Mental Health (NIMH). The project was carried out by principal investigators from the University of North Carolina, Duke University, the University of Southern California, the University of Rochester, and Yale University in association with Quintiles, Inc., the program staff of the Division of Interventions and Services Research of NIMH, and investigators from 56 sites in the United States (CATIE Study Investigators Group). AstraZeneca Pharmaceuticals L.P., Bristol-Myers Squibb Company, Forest Pharmaceuticals, Inc., Janssen Pharmaceutica Products, L.P., Eli Lilly and Company, Otsuka Pharmaceutical Co., Ltd., Pfizer, Inc., and Zenith Goldline Pharmaceuticals, Inc., provided medications for the studies. The Foundation of Hope, Raleigh, North Carolina, also supported this work. Details about the CATIE Study Investigators Group are available at www.catie.unc.edu/schizophrenia/locations.html#clinicalsitelocation list.