In the absence of a safe and effective treatment for tardive dyskinesia, recognition of risk factors is important. There is a relatively good consensus about some demographic and clinical risk factors, such as age, and some degree of consensus about other risk factors, such as female gender, brain damage, diabetes mellitus, and early development of extrapyramidal side effects (
1–
3). However, the relative contributions of various medication variables are far from clear (
1,
2).
On the basis of the literature it was hypothesized that the cumulative amount of neuroleptic medication, the number of interruptions in neuroleptic treatment, and the cumulative amount of anticholinergic medication would increase the risk of tardive dyskinesia (
1,
2). Intermittent neuroleptic treatment lowers the cumulative amount of neuroleptics. In the 1970s such treatment was advocated as a strategy for reducing the risk of tardive dyskinesia (
4,
5). The results of the few studies that addressed this issue are not consistent. Jeste et al. (
6) reported drug-free intervals as a risk factor for persistent tardive dyskinesia. However, their results were based on a small number of patients, all older than 50 years. Two studies with larger study groups did not indicate that drug-free periods were a risk factor (
7,
8). A few studies have compared intermittent with continuous neuroleptic treatment. Some showed no difference, while others showed a higher risk of tardive dyskinesia in the group with intermittent neuroleptic treatment (
1,
2,
4,
9). However, these results pertain to highly selective study groups, which makes generalizations difficult. In our study group the selection bias was reduced because our subjects were all psychiatric inpatients from a well-defined area (
10).
The goal of our study was to examine the association between the three lifetime medication variables (cumulative amount of neuroleptics, number of interruptions in neuroleptic treatment, and cumulative amount of anticholinergics) and tardive dyskinesia.
METHOD
The study was performed in the only psychiatric hospital on Curaçao, which is the main island of the Netherlands Antilles. The target group comprised all inpatients in this hospital on June 1, 1992 (
10). The population is mainly of Afro-Caribbean origin. To be eligible for the study a patient had to fulfill the following criteria: 1) informed consent, 2) no organic disorders that could cause movement disorders, 3) history of neuroleptic use for at least 3 months, and 4) current use of neuroleptics. The last criterion was used because it was impossible to find out whether a patient was suffering from tardive dyskinesia at the time when neuroleptic treatment was discontinued. The inclusion criteria were fulfilled by 166 patients.
The psychiatric diagnoses (DSM-III-R) were based on the chart notes and confirmed in a consensus meeting between one of us (P.N.V.H.) and the treating psychiatrist. Tardive dyskinesia was assessed with the Abnormal Involuntary Movement Scale (AIMS) (
11), and cases were defined according to Schooler and Kane's criteria (
12). The sum of scores on the first seven items of the AIMS was used as a rating of the severity of tardive dyskinesia.
The lifetime medication data were reviewed by two junior nonpsychiatric physicians who had no knowledge of the existence of movement disorders in the patients. All medication data for a patient could be found in one file and had been recorded by physicians trained in Europe or the United States. The junior physicians were instructed to mark missing or puzzling data. Lifetime medication data were considered valid if they were clearly indicated in the chart notes for at least 90% of the time during which medication had been taken. Neuroleptic doses were converted into chlorpromazine equivalents, and doses of anticholinergic medication were converted into benztropine equivalents (
13).
For each of the three lifetime medication variables a separate multiple logistic regression analysis and multiple linear regression analysis were applied, and the following potential confounders were incorporated: age, sex, diagnosis (schizophrenia yes/no), diabetes mellitus, leukotomy, current dose of neuroleptics, and current use of anticholinergics, lithium, antidepressants, benzodiazepines, and other medication (
1,
14). The resulting odds ratios and regression coefficients can be interpreted as measures of the effects of the risk factors corrected for all potential confounders.
RESULTS
The lifetime medication data were considered valid for 133 (80.1%) of the patients. The characteristics of the 133 patients were as follows: male, 73.4% (N=98); diagnosis of schizophrenia, 84.2% (N=112); diabetes mellitus, 11.3% (N=15); leukotomy, 12.8% (N=17); mean age=51.5 years (SD=15.3) (men: mean age=49.4 years, SD=14.2; women: mean age=57.3 years, SD=16.8). The medications used at the time of the assessment consisted of anticholinergics (34.6%, N=46), benzodiazepines (18.0%, N=24), antidepressants (4.5%, N=6), lithium (10.5%, N=14), and others (21.8%, N=29). The current mean neuroleptic dose was 717 mg of chlorpromazine equivalents (SD=748) (men: mean dose=724, SD=700; women: mean dose=700, SD=879). The prevalence of tardive dyskinesia was 36.1% (N=48).
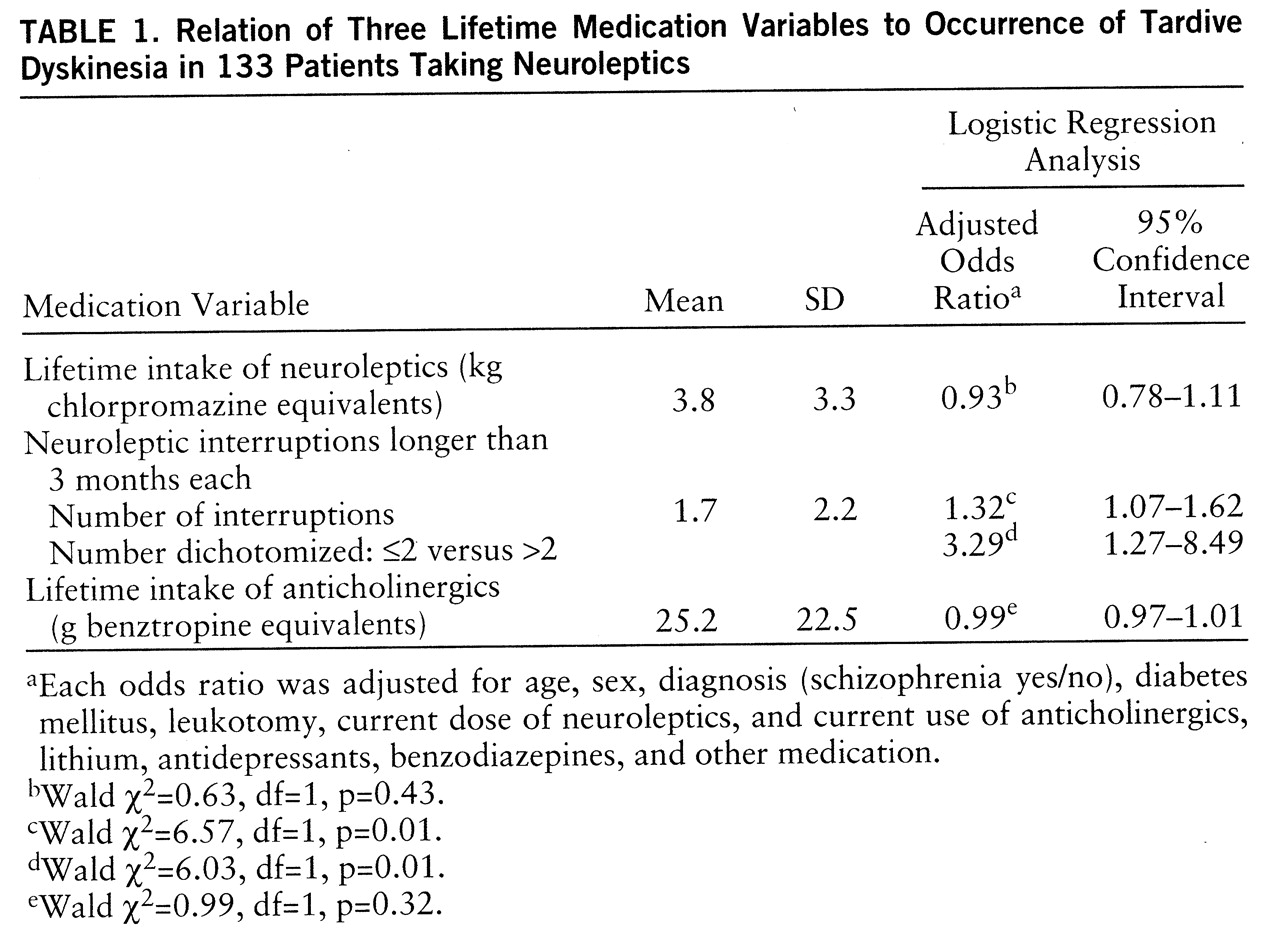
Table 1 shows the relationship between the three lifetime medication variables and the occurrence of tardive dyskinesia. Only the number of neuroleptic interruptions was significantly related to the occurrence of tardive dyskinesia. When the number of neuroleptic interruptions was dichotomized, into two or fewer and more than two, the resulting adjusted odds ratio was 3.29. The linear regression analysis, which determined the relation of each of the three lifetime medication variables to the severity of tardive dyskinesia, showed no significant effect for the lifetime intake of neuroleptics (β=–0.17, t=–1.29, df=120, p=0.20) or the lifetime intake of anticholinergics (β=–0.03, t=–1.91, df=120, p=0.06) but did show a significant effect for the number of neuroleptic interruptions (β=0.36, t=2.43, df=120, p=0.02).
DISCUSSION
The most important finding is that intermittent neuroleptic treatment increases the risk of tardive dyskinesia. This finding supports the schizophrenia protocol involving long-term low-dose neuroleptic treatment rather than targeted or intermittent neuroleptic treatment.
Our finding is in line with the results of animal studies (
4); for instance, one study of rats (
15) showed that after neuroleptic withdrawal it was only the intermittently treated group that developed persistent increases in vacuous chewing movements. It could be that repeated “on-off” manipulations of some dopaminergic systems may have a greater negative impact than continuous dopaminergic activity.
Our finding that intermittent neuroleptic treatment is a risk factor for tardive dyskinesia is further supported by the fact that it was a risk factor not only for the presence but also the severity of tardive dyskinesia. Furthermore, when our data were analyzed with the use of stepwise logistic regression in which a stepwise algorithm generated the “best” model for predicting tardive dyskinesia (
7,
8,
14), the number of interruptions in neuroleptic treatment turned out to be the second factor after the well-known risk factor of age.
Our inability to find a relationship between the cumulative amount of neuroleptics used and tardive dyskinesia may be due to the long duration of neuroleptic treatment for most of the patients of our study group; only seven patients had used neuroleptics for less than 2 years. It may be that cumulative dose as a risk factor for tardive dyskinesia is important only during the first few years of neuroleptic treatment (
1,
2,
4).
The fact that we found no relationship between the cumulative amount of anticholinergics and tardive dyskinesia is consistent with the results of investigators who have suggested that anticholinergics increase the severity of existing tardive dyskinesia but do not cause tardive dyskinesia (
1,
2).
This study has some limitations. First, the number of interruptions in treatment with neuroleptics was measured retrospectively; therefore, the resulting measurement error could produce a bias. However, all medication data for each patient were noted in one file, mainly by western-trained physicians. Second, 33 patients were excluded from the analysis because no valid lifetime medication data were available for them. For this group, the prevalence and the severity of tardive dyskinesia were the same as for the study group, which makes it less likely that the inclusion of this group would have changed the results significantly. Third, a retrospective study of risk factors is particularly vulnerable to selection biases. However, the inclusion of nearly all psychiatric inpatients in a circumscribed area reduces the selection bias considerably. Fourth, it could be that in some patients the expression of tardive dyskinesia was masked by the neuroleptics. However, this is inevitable for any prevalence study in which patients are taking neuroleptics. Fifth, patients not currently using neuroleptics were excluded because no data about tardive dyskinesia were available at the time neuroleptic treatment was discontinued. However, if this group had been added to the analysis (and the current status of tardive dyskinesia was used), the results would not have changed significantly.
In conclusion, if treatment with neuroleptics is required, the treatment should preferably not be given intermittently; the risk of tardive dyskinesia is three times as great for patients with more than two interruptions as for patients with two or fewer interruptions.