The use of pharmacological challenges in patients with panic disorder is unique in that the leading clinical phenomenon, the panic attack, can be provoked and quantified in a laboratory setting. Numerous studies have corroborated the finding of Pitts and McClure (
1) that in about 70% of patients with panic disorder, sodium lactate is a reliable chemical agent for experimentally inducing panic attacks (
2). Because the benzodiazepine receptor antagonist flumazenil also produces panic attacks in panic disorder patients but not in control subjects (
3,
4), Nutt and co-workers (
3) have suggested that there is a shift in the benzodiazepine receptor “set point” toward an inverse agonistic action of flumazenil in patients with panic disorder. In the present study we compared sodium lactate and flumazenil in patients with panic disorder to determine whether comparable panicogenic effects can be exerted through different mechanisms in the same subjects. If flumazenil exerts anxiogenic activity because of inverse agonistic action, there should also be an activation of the hypothalamic-pituitary-adrenal (HPA) system, in contrast to the effects of lactate (
2), and an increased heart rate, as seen with β-carbolines (
5,
6).
METHOD
Ten patients (four women and six men; mean age=31.6 years, SD=9.2) with a diagnosis of panic disorder, but without a comorbid axis I disorder as assessed with the Structured Clinical Interview for DSM-III-R (
7), were studied. The subjects had been medication free for at least 10 days and had undergone thorough medical examination to rule out other illnesses, drug intake, and lifestyles that could interfere with the study. The protocol was approved by the ethics committee for human experiments of the Max Planck Institute of Psychiatry, Munich. After complete description of the study to the subjects, written informed consent was obtained.
All subjects were studied from 9:00 a.m. to 1:00 p.m. in a supine position in a soundproof room with a single bed. In a double-blind randomized design, each subject received an intravenous infusion (10 ml/kg of body weight) of 0.5 M sodium lactate, 0.9% saline with a 2-mg flumazenil bolus at 11:00 a.m. (20 ml injected within 1 minute), or 0.9% saline alone (placebo) for 20 minutes, from 11:00 to 11:20 a.m. The infusions were administered on separate days, with at least 2 days between experimental conditions. The Acute Panic Inventory (
8) was administered at 11:00, 11:10, 11:20, and 11:30 a.m. by a rater blind to the procedure; the maximum intensity during the observation period was noted. For a decision that a panic attack had occurred, an Acute Panic Inventory total score exceeding 20 and an increment of at least 14 points over the preinfusion score were required. Plasma cortisol and ACTH concentrations were determined with commercial radioimmunoassays. Heart rate was measured by an automatic device each time the psychopathology rating was made.
Panic attack frequencies in the two active treatment conditions were compared by means of the McNemar paired chi-square test. For statistical comparison of mean Acute Panic Inventory scores, hormonal concentrations, and heart rate between the saline, lactate, and flumazenil conditions and between the various time points, analysis of variance (ANOVA) with a repeated measures design was performed. Treatment (three levels) and time (four levels) were the two within-subject factors. Hypothesis tests for the main effects and interactions of these factors were based on multivariate criteria such as Wilks's lambda and its approximated F value. When significant main effects or interactions were found, tests with contrasts followed in an ANOVA to locate the levels of the factor(s) with significant differences in the means of the variables. The contrasts (group mean differences) were tested for significance with approximated F values of the Wilks's lambda statistics. For variance homogeneity, the variables of plasma hormonal concentrations used in the analysis were transformed with the log transformation (x*=ln[x]) before analysis. Alpha=0.05 was accepted as a nominal level for significance. To keep the type I error less than 0.05, all post hoc tests (tests with contrasts) were performed at a reduced level of significance (adjusted alpha accord~ing to the Bonferroni procedure). The power of the study—i.e., the probability with which differences in the considered variables between the various factor levels with values greater than 77% of the pooled standard deviations will be identified as significant—was set at 0.80.
RESULTS
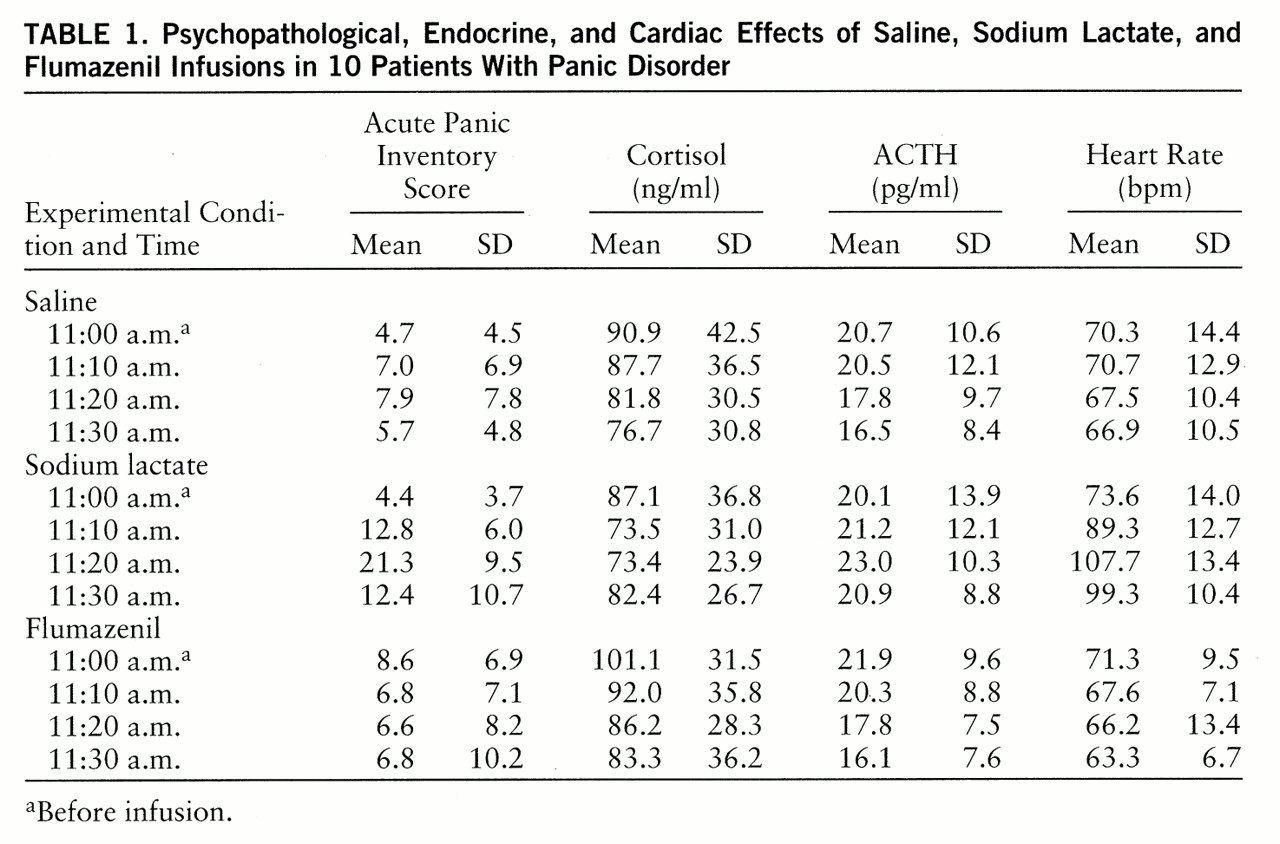
None of the 10 patients experienced a panic attack in the flumazenil or saline (placebo) conditions, but eight of the 10 patients did so during the lactate condition (p<0.05, McNemar's test of significance). As expected, psychopathology as assessed with the Acute Panic Inventory was significantly influenced by treatment and by time as well as by their interaction (Wilks's multivariate tests of significance; effect of treatment: approximated F=5.19, df=2,8, p=0.04; effect of time: approximated F=10.52, df=3,7, p=0.007; interaction of treatment and time: approximated F=52.24, df=6,4, p=0.001). Tests with contrasts between the various time points within each of the different treatments and between the various treatments at each of the time points showed that the Acute Panic Inventory score was influenced significantly by lactate only. Lactate infusion resulted in significantly higher Acute Panic Inventory scores at 11:20 a.m. than did the other treatments (tests with contrasts in ANOVA; for F values, df=2,13, p<0.05). Compared to baseline scores, lactate-treated subjects had significantly higher Acute Panic Inventory scores at 11:10 a.m., 11:20 a.m., and 11:30 a.m. (tests with contrasts in ANOVA; for F values, df=3,12, p<0.05). No significant changes over time were observed after either flumazenil or saline administration (
table 1).
Neither treatment nor time had significant main effects on plasma cortisol and ACTH concentrations. However, their interaction influenced cortisol concentrations significantly (Wilks's multivariate test of significance; approximated F=10.02, df=6,4, p=0.02) and showed a tendency to influence ACTH concentrations. Cortisol levels were decreased at 11:10 a.m. and 11:20 a.m. in the lactate condition and at 11:20 a.m. and 11:30 a.m. in the flumazenil condition, but compared to the baseline levels the differences were not significant. Compared to the baseline values, there was a significant decrease in ACTH concentrations during the saline and flumazenil conditions at 11:30 a.m. (tests with contrasts in ANOVA; for F values, df=3,12, p<0.05).
In contrast, heart rate was significantly influenced by treatment and time and by their interaction (Wilks's multivariate tests of significance; effect of treatment: approximated F=67.14, df=2,8, p=0.001; effect of time: approximated F=13.69, df=3,7, p=0.003; interaction of treatment and time: approximated F=25.41, df=3,7, p=0.004). The main effects were caused by an increase in heart rate throughout the entire lactate condition after the challenge had been started (tests with contrasts in ANOVA; for F values, df=2,13, p<0.05). In addition, there was a decrease in heart rate at 11:30 a.m. after flumazenil administration (tests with contrasts in ANOVA; for F values, df=3,12, p<0.05).
It should be noted that for none of the variables studied was there any order effect; i.e., the responses to flumazenil and lactate were equivalent whether the infusion was the first, second, or third.
DISCUSSION
The major finding of our study was that patients with panic disorder who responded to sodium lactate with typical psychopathological changes did not experience anxiogenic or panic-provoking effects after administration of the benzodiazepine receptor antagonist flumazenil. In addition, neither hormonal alterations nor changes in heart rate indicated that flumazenil exerts an inverse agonistic activity in such patients. We were unable to replicate the findings of Nutt et al. (
3).
Because a panicogenic activity of flumazenil was reported in 12 of 20 patients with panic disorder (
3,
4), the negative findings of our study raise the question of whether our lactate-sensitive subjects represented a special subgroup. This seems to be unlikely, however, since the responsiveness to different pharmacological provocations in the same patients has been described as comparable (
9–
12), and the observed rate of lactate-induced panic attacks in our study group was within the reported range (
2). The failure to confirm flumazenil's panicogenic effects in patients with panic disorder also cannot be explained sufficiently by differences in the experimental design or measurement techniques. Comparison of ratings, rating frequency, and duration of drug-free periods did not reveal prominent differences. However, although Nutt et al. (
3) described a 1-hour control period and randomization of the order of drug administration, separation of experimental days may be regarded as a prerequisite for a placebo condition. Our results suggest that the hypothesized shift in the benzodiazepine receptor set point (
3) cannot be generalized to patients with panic disorder.
The proposed shift in the benzodiazepine receptor set point and the suggested inverse agonistic activity of flumazenil in patients with panic disorder (
3) encouraged us to hypothesize that flumazenil-induced panic attacks would be accompanied by an activation of the HPA system as seen with β-carbolines (
6). In our study group, the unchanged or reduced plasma cortisol and ACTH concentrations did not support an inverse agonistic activity of flumazenil in patients with panic disorder. Heart rate was increased after sodium lactate infusion, whereas there was a significant decrease 30 minutes after the infusion of flumazenil. Furthermore, the lack of an increase in heart rate soon after flumazenil administration argues against an inverse agonistic activity of this substance in our group of patients. The lack of any behavioral effects could explain why heart rate was influenced in a direction opposite to that found by Nutt et al. (
3).
In summary, our findings regarding behavioral, endocrine, and heart rate changes do not lend support to the view that there is a shift in the benzodiazepine receptor set point in patients with panic disorder who are sensitive to sodium lactate. The failure to confirm flumazenil's panicogenic activity should further stimulate reconsideration of a theory about an altered benzodiazepine-GABAA receptor function in panic disorder.