Exaggerated startle is listed as a symptom of posttraumatic stress disorder (PTSD) in DSM-IV. Nevertheless, empirical studies have not consistently found elevated startle reactivity in individuals with PTSD (
1–
8). These mixed results regarding exaggerated startle in the context of the extensive historical clinical impressions of elevated startle reactivity in PTSD (
9) underscore the need for additional studies that would establish the circumstances under which exaggerated startle responses are seen in PTSD. In addition, animal models of stress-induced alterations of startle provide a framework to better understand the causes of, as well as the neurobiological mechanisms associated with, abnormal startle responses in PTSD (
10,
11).
Results from Vietnam veterans with PTSD suggest that the symptom of exaggerated startle may reflect an enhanced anxiogenic reaction to an aversive situation. In a previous study (
5), we found normal baseline startle in response to a nonstressful procedure in a group of Vietnam veterans with PTSD. However, when we implemented a stressful procedure in a different group of veterans with PTSD (
12), we found that startle was exaggerated. These results suggested that experimental stressors (independent of trauma-related cues) play a determining role in the abnormal startle reactivity in PTSD.
We found further support for this hypothesis in a recent study using a within-subject procedure (
13). Vietnam veterans with PTSD had normal baseline startle during an initial test performed without explicit experimental stress, but they showed elevated startle throughout a second testing procedure that involved the administration of unpleasant electric shocks. During this latter procedure, participants were told that they could receive a shock during threat periods but not during safe periods. Startle was elevated in the veterans with PTSD even before the shock electrodes were attached to their wrist and during the safe periods, when no shocks could be administered. These results indicate that “stressful” experimental contexts induce an upward shift in baseline startle levels in individuals with PTSD. Of note, the magnitude of the increase in startle during threat periods (fear-potentiated startle) did not differ significantly in individuals with and without PTSD. This differential fear response to explicit (e.g., the threat signal) and contextual (e.g., the experimental room) stimuli suggests that veterans with PTSD might be hypersensitive to some, but not all, stressors. These results are heuristically advantageous because they provide clues to the neurobiology of PTSD.
Preclinical investigations indicate that different brain structures may be involved in explicit versus contextual fear or long-term sensitization (
14–
17). Using elevation of the acoustic startle reflex in rats as a marker for fear or anxiety, it has been found that lesions of the amyg~dala block the acquisition of fear in response to explicit stimuli (
17) but that lesions of the bed nucleus of the stria terminalis do not (
16). On the other hand, lesions of either structure block the gradual increase in baseline startle that develops over successive days of fear conditioning—an effect that may represent either context conditioning or long-term sensitization—but not fear to explicit cues (
16). Moreover, chemical inactivation of either the amygdala (
18) or the bed nucleus of the stria terminalis (McNish and Davis, unpublished observation) blocks or attenuates contextual fear conditioning. Thus, the bed nucleus of the stria terminalis is involved in contextual fear and/or long-term sensitization, but not in conditioned fear to an explicit cue.
The hypothesis that veterans with PTSD are abnormally sensitive to aversive contexts would be further strengthened by replicating the above findings using a different procedure (i.e., a different aversive context). We have developed a new procedure to examine the effect of contextual stimuli on startle using changes in background illumination as a way to alter the experimental context (
19,
20). In humans, a diurnal species, the magnitude of startle is greater in the dark than in the light (
19). This effect is diminished by diazepam (our unpublished observation). In contrast, in rats, a nocturnal species, the magnitude of startle is increased by bright lights. This latter effect is blocked by buspirone (
20), and the facilitation of startle by bright lights in the rat is prevented by inactivation of the bed nucleus of the stria terminalis (
21). Taken together, these results suggest that a similar mechanism, dependent on the bed nucleus of the stria terminalis, may be involved in fear in response to contextual stimuli or long-term sensitization, as well as in the aversive response to changes in illumination.
In the present study we examined the facilitation of startle by darkness in veterans with and without PTSD and in civilians without PTSD. We hypothesized that if individuals with PTSD are abnormally sensitive to contextual fear, their facilitation of startle in the dark should be greater than that of individuals without PTSD. The study also provided the opportunity to examine prepulse inhibition of startle, the inhibition of startle by a weak prestartle stimulus. At the present time it is unclear whether prepulse inhibition is normal or abnormal in PTSD (
2,
5–
7).
METHOD
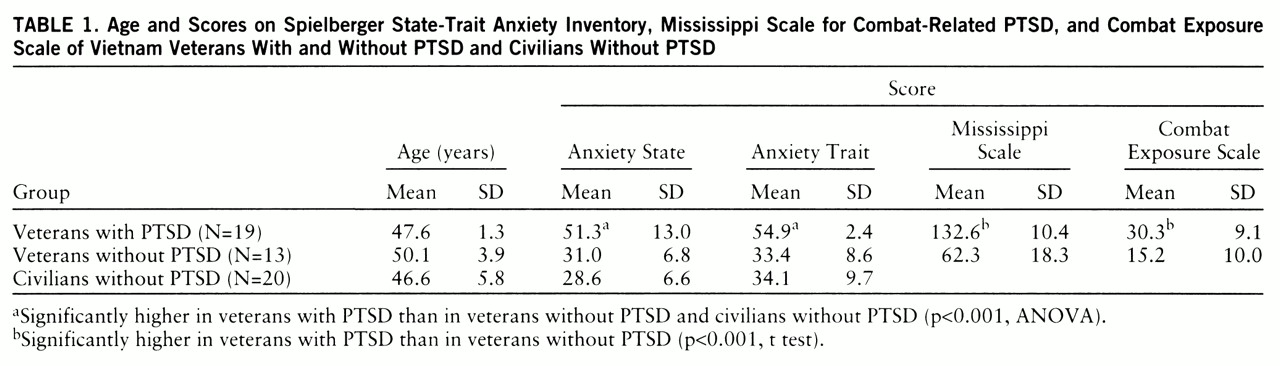
The subjects included 21 treatment-seeking Vietnam combat veterans with PTSD. All patients had been free of medication for at least 1 month before testing. The comparison subjects were 15 Vietnam combat veterans and 22 civilians without PTSD. Two participants in each group were excluded from the data analysis because they had virtually no startle eye blink. The final study groups consisted of 19 veterans with PTSD, 13 veterans without PTSD, and 20 civilians without PTSD. The groups did not differ significantly in age (
table 1). The combat and civilian comparison subjects were screened for medical and psychiatric illnesses by staff of the Neurobiological Study Unit of the National Center for PTSD. After complete description of the study, written informed consent was obtained from all subjects.
All patients met criteria for PTSD according to their responses to the Structured Clinical Interview for DSM-III-R (SCID) (
22). Patients with any major medical illness, organic brain syndrome, schizophrenia, bipolar disorder, or current substance abuse/dependence were excluded from study. Combat history of all the veterans was verified by military discharge forms. The comparison subjects had no current major medical problems or psychiatric disorders according to their responses to the SCID—Non-Patient Version (
23).
Toxicology screenings confirmed that the participants had been free of psychotropic drugs or illicit substances for at least 1 month before testing. Participants who showed hearing deficits in the 1000–3000-Hz range on the Welsh Allen audioscope were excluded from the study.
Trait and state anxiety were investigated by using the Spielberger State-Trait Anxiety Inventory (
24). The Mississippi Scale for Combat-Related PTSD (
25) and the Combat Exposure Scale (
26) were used to assess the intensity of PTSD symptoms and the level of combat exposure, respectively.
The recording took place in a sound-attenuated chamber. The startle reflex was recorded with a commercial startle system (San Diego Instrument). Acoustic stimuli were delivered binaurally through headphones (Telephonics model TDH-39P) in the absence of background noise. Sound intensities were calibrated with a Quest sound level meter (Model 215). The startle stimulus was a 40-msec-duration 103-dB(A) burst of white noise with an instantaneous rise-time. It was delivered alone (pulse alone) or preceded by a 30-msec 70-dB(A) white noise prepulse (prepulse plus pulse). The onset of the prepulse was presented 120 msec before the onset of the startle stimulus. These stimuli were presented in complete darkness or in the presence of light. In the light condition, the room was illuminated with a 60-watt bulb located approximately 2 m in front of the subjects. The experiment started with a startle adaptation procedure consisting of two blocks of four pulse-alone trials to reduce initial startle reactivity. The startle adaptation procedure was performed in the dark with approximately half of the subjects in each group and in the light with the remaining subjects. This procedure was immediately followed by two alternating dark and light phases (i.e., dark/light/dark/light or light/dark/light/dark). The illumination of the adaptation procedure and of the first dark/light phase were opposite (e.g., adaptation in the dark was followed by a light phase). There were no breaks between the adaptation procedure and the first dark/light phase and between dark and light phases. Two pulse-alone and two prepulse-plus-pulse trials were delivered irregularly during each of the dark/light phases. The interval between startle trials varied from 18 to 25 seconds.
The eye blink component of the startle reflex was measured by recording activity from the orbicularis oculi muscle below the left eye with two gold disk electrodes (Grass Instrument). The ground electrode was placed on the left arm. Impedance was kept below 5 kilohms. The electromyographic (EMG) activity was filtered (1–500 Hz), digitized at 1 kHz for 250 msec from the onset of the acoustic stimuli, and stored for off-line analysis. A 60-Hz notch filter was used to eliminate 60-Hz interference.
The method of analysis of the blink reflex has been presented in detail elsewhere (
27). Briefly, following amplification (San Diego Instrument) and digital filtering of the EMG signal with a 20.9-Hz low-pass filter, peak amplitude of the blink reflex was determined by using a program derived from Balaban et al. (
28). This program eliminates trials with unstable baselines and was set to detect peak responses in a 21–100-msec window after startle onset. It calculates the average muscle tension for the 20-msec interval after startle onset and before the reflexive eye blink. This average value is used as the base value from which the peak response is computed. This value is used because it is temporally close to the reflex eye blink and should therefore be a good estimate of the relationship between base tension and eye blink amplitude. In addition, because the program detects and omits trials in which an eye blink occurs during the base interval, this measure is relatively uncontaminated by spontaneous eye blinks. The number of rejected trials was very low (less than 1%) and did not differ significantly among groups and between phases.
The data analysis was performed with mixed-model analyses of variance (ANOVAs). To analyze the startle response in the adaptation procedure, the magnitude of the eye blink was averaged within blocks. These data were entered in a three-way ANOVA with group (veterans with PTSD, veterans without PTSD, civilians without PTSD) and illumination during adaptation (dark, light) as between-subject factors and blocks (two) as a within-subject factor. Startle responses during the dark/light phases were averaged within phases separately for each block. Group comparisons were performed with a four-way ANOVA with group (veterans with PTSD, veterans without PTSD, civilians without PTSD) and order (dark phase first, light phase first) as between-subject factors, and phase (dark, light) and block (two) as within-subject factors. Because no prepulse trials were presented during the adaptation procedure, prepulse inhibition was investigated only during the light/dark phases.
Prepulse inhibition in subjects with small eye blinks is difficult to interpret; therefore, subjects with eye blinks of less than 25 µV in response to pulse-alone trials were not included in the analysis. This procedure resulted in the exclusion of three veterans with PTSD, four veterans without PTSD, and two civilians without PTSD. The amplitudes of the eye blink in response to pulse-alone and prepulse-plus-pulse trials were averaged within phases separately for each block. Prepulse inhibition was then calculated as percent change of the magnitude of the eye blink in response to pulse-alone trials to magnitude of the eye blink in response to prepulse-plus-pulse trials, i.e., (pulse–prepulse)/pulse×100. Greater percent scores were associated with greater prepulse inhibition. Prepulse inhibition scores were analyzed by using the same three-way ANOVA as for the pulse-alone scores. Post hoc analyses of significant main group effects or interactions with group were done by comparing the veterans with PTSD with 1) the civilians without PTSD and 2) the veterans without PTSD in separate analyses to assess whether differences in startle modulation were due to PTSD per se or combat exposure. All tests were two-tailed.
Pearson correlations were also performed between startle measures reflecting group differences (see Results section) and two scores derived from the Mississippi scale: the total score and an arousal score based on a subset of questions 3, 5, 20, 24, 25, 27, and 31 (Keane, personal communication). The arousal score was selected because startle is listed in the hyperarousal cluster in DSM-IV.
RESULTS
Trait and state anxiety (
table 1) differed significantly among the three groups (F=27.0, df=2,49, p<0.001, and F=29.4, df=2,49, p<0.001, respectively). Veterans with PTSD had significantly higher trait and state anxiety than the veterans without PTSD (F=36.9, df=1,49, p<0.001, and F=51.4, df=1,49, p<0.001, respectively) and civilians (F=44.9, df=1,49, p<0.001, and F=33.5, df=1,49, p<0.001, respectively). Mississippi scale and Combat Exposure Scale scores (
table 1) were significantly higher in the veterans with PTSD than in the veterans without PTSD (t=12.8, df=30, p<0.001, and t=9.0, df=30, p<0.001, respectively).
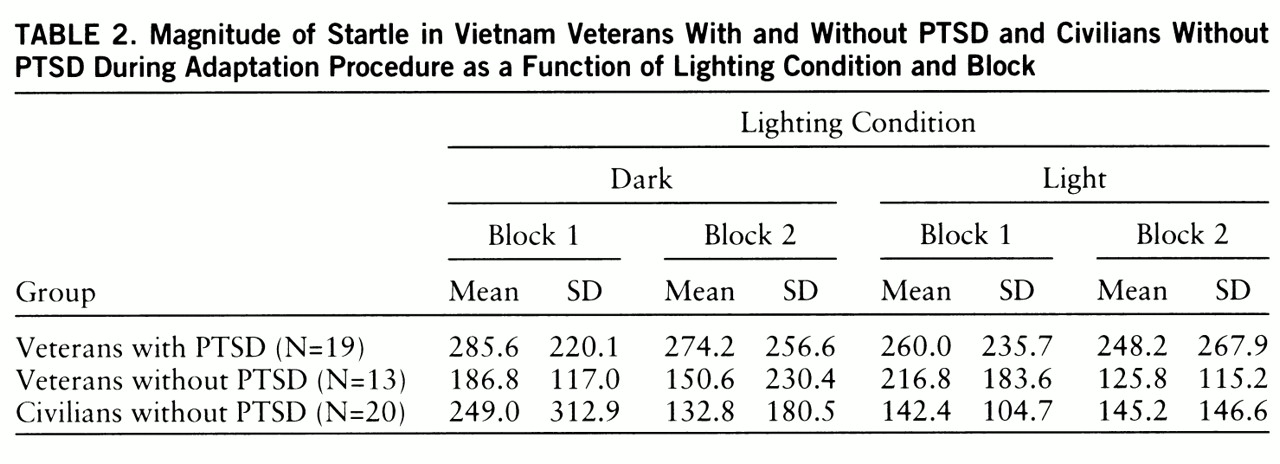
Startle magnitude during the adaptation procedure (
table 2) did not differ significantly among groups (F=2.6, df=2,46, p<0.09). There was a significant group-by-block-by-illumination interaction (F=3.8, df=2,46, p<0.03). This interaction was mainly due to the fact that in the civilians without PTSD, startle magnitude was greater when adaptation was performed in the dark than in the light in block 1 but not in block 2. Within-group analyses indicated a significant block-by-illumination interaction in the civilians without PTSD (F=3.8, df=2,46, p<0.03) but not in the two other groups.
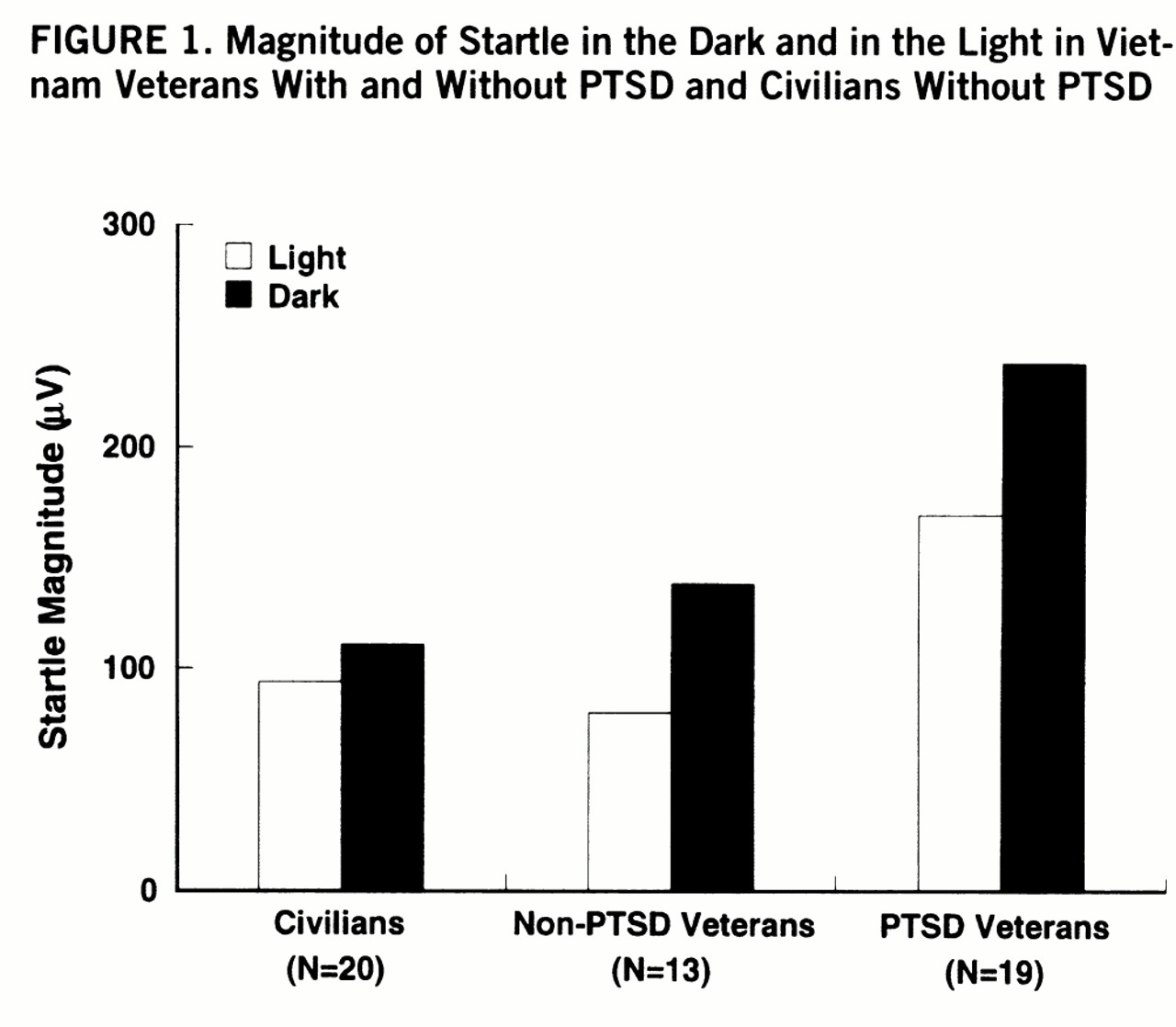
Figure 1 shows the magnitude of startle during the light/dark phases in each group. There were significant main effects of group (F=4.2, df=2,46, p<0.03) and phase (F=3.9, df=2,46, p<0.03) as well as a significant group-by-phase interaction (F=3.9, df=2,46, p<0.03). Darkness increased startle in each group (veterans with PTSD: F=32.5, df=1,46, p<0.001; veterans without PTSD: F=19.5, df=1,46, p<0.001; civilians without PTSD: F=9.5, df=1,46, p<0.01), but the magnitude of this facilitation was greater in the veterans with PTSD than in the civilians without PTSD (F=7.1, df=1,46, p<0.01). However, the increase in startle in the dark did not differ significantly between the veterans with and without PTSD (F=0.22, df=1,46, n.s.).
Furthermore, startle was significantly greater in the veterans with PTSD than in the veterans without PTSD and the civilians without PTSD in the light phase (F=5.2, df=1,46, p<0.04, and F=4.4, df=1,46, p<0.05, respectively) and in the dark phase (F=4.4, df=1,46, p<0.05, and F=9.0, df=1,46, p<0.01, respectively).
To examine whether the differential facilitation of startle in the dark among the three groups was due to differences in baseline startle, the magnitude of startle facilitation in the dark (startle magnitude in the dark minus startle magnitude in the light) was compared among the groups by using a one-way analysis of covariance. The magnitude of startle in the light was used as a covariate. The group main effect was significant (F=3.3, df=2,48, p<0.05), indicating differential startle facilitation in the dark among groups.
In the veterans with and without PTSD combined, there were significant correlations between the magnitude of startle in the light phase and 1) total Mississippi scale scores (r=0.36, df=30, p<0.05) and 2) arousal scores derived from the Mississippi scale (r=0.45, df=30, p<0.01). However, the facilitation of startle in the dark (difference scores between magnitude of startle in the dark minus magnitude of startle in the light) was not significantly correlated with any of these scores.
Prepulse inhibition was not significantly affected by darkness and did not significantly differ between the veterans with PTSD (mean=46.4%, SD=37.5%), the veterans without PTSD (mean=65.2%, SD=37.4%), and the civilians without PTSD (mean=48.3%, SD=41.4%).
DISCUSSION
The main results of the present study were 1) that the overall magnitude of startle was greater in the veterans with PTSD than in the veterans and civilians without PTSD and 2) that the facilitation of startle by darkness was enhanced in the combat veterans with PTSD compared with the civilians without PTSD but not compared with the veterans without PTSD. The finding that startle was increased by darkness replicates earlier results in a large sample of healthy young adults (mean age=21 years) (
19). The participants in the present study were in their late 40s and early 50s, suggesting that the facilitation of startle by darkness occurs across a wide age range.
The facilitation of startle by darkness was enhanced in the veterans with PTSD compared with the civilians without PTSD. The failure to find a similar effect when comparing the two groups of veterans was unexpected. The hypothesis that startle would be facilitated to a greater extent in individuals with PTSD than in those without PTSD was suggested by 1) the finding that individuals with PTSD are hypersensitive to stressful context (
13) and 2) preclinical data indicating that the same brain structure (i.e., the bed nucleus of the stria terminalis) may be involved in fear in response to contextual stimuli or long-term sensitization (
16) and in the facilitation of startle by changes in illumination (
21). Given that veterans without PTSD have not been shown to be abnormally sensitive to contextual stimuli in previous studies (
13), it is unlikely that their enhanced facilitation of startle in the dark in the present study was caused by enhanced contextual fear. Rather, it is possible that during their combat experiences, Vietnam veterans became aversively conditioned to darkness. According to this interpretation, the enhanced facilitation of startle in the dark in the combat veterans would be a conditioned emotional response to a stimulus reminiscent of wartime experience or military training. In other words, in the present study darkness might have been both a contextual stimulus and an explicit stimulus.
The difference between these two types of stimuli can be clarified by preclinical studies. In rats, inactivation of the bed nucleus of the stria terminalis, but not the amygdala, blocks the ability of unconditioned bright lights (i.e., contextual stimuli) to facilitate startle (
21). However, when a light is used as an explicitly conditioned stimulus by virtue of pairing it with shock, its ability to increase startle is blocked by inactivation of the amygdala but not the bed nucleus of the stria terminalis (
21). These results suggest that the enhanced facilitation of startle in the dark in the combat veterans may not implicate the bed nucleus of the stria terminalis. Rather, it may reflect the activation of an amyg~dala-mediated conditioning process in response to a powerful affective stimulus.
An alternate explanation is that aversive responses to contextual stimuli are not uniform processes mediated by a single brain structure. Different types of contextual stimuli or different response measures may involve different brain structures. For example, the hippocampus has been implicated in contextual fear conditioning when freezing in rats was used as a measure (
14,
15) but not when facilitation of startle was studied (
29). Moreover, the role of the hippocampus in the facilitation of startle by changes in illumination is unknown. Some of these brain structures might be more sensitive to trauma exposure, whereas others might be more affected by PTSD per se. Research on contextual fear in animals is still in its infancy, and it has been largely ignored in humans. Studies such as the present one should serve as an impetus for this type of research.
The finding of greater startle facilitation in the dark in combat veterans than in civilians is also consistent with clinical experience. Hypervigilant combat veterans frequently report poor sleep and fear of the night. With the onset of darkness, it becomes increasingly difficult to monitor the environment and to feel safe. As one veteran put it, “The only time I really sleep is at daybreak. I hate darkness and the night. I just can't stay asleep. Every little noise bothers me. And even if I do sleep, it's like I sleep with one eye open. When morning comes, I can relax and go to sleep.” The present results raise the possibility that these types of difficulties might not be restricted to veterans with PTSD, but may characterize veterans without PTSD as well.
In addition to the finding of greater facilitation of startle in the dark in veterans with and without PTSD than in civilian subjects without PTSD, the present study also found that overall startle was elevated only in the veterans with PTSD. This exaggerated startle response is consistent with clinical observations and with the hyperarousal cluster symptoms of PTSD (DSM-IV). However, empirical studies have provided inconsistent results. Increased (
2,
3,
7,
8), normal (
1,
4), or even reduced (
5) baseline startle have been reported in individuals with PTSD. One possibility is that exaggerated startle characterizes only a subgroup of veterans with PTSD—those with high arousal or with more severe symptoms. This is suggested by the positive correlation that was found between startle magnitude and the total score as well as the arousal subscore of the Mississippi scale.
Alternatively, exaggerated startle in the veterans with PTSD could be explained by the aversive nature of the experimental context. In our laboratory, we have found abnormal baseline startle only in individuals with recent-onset PTSD (i.e., Gulf War veterans [3] and women recently exposed to sexual assault [2]). Individuals with longstanding PTSD exhibit normal baseline startle (
5), except under stressful testing conditions (
2,
30).
Prepulse inhibition was not found to differ significantly among groups. Prepulse inhibition has been found to be normal (
2,
7) or reduced (
6) in PTSD. We have reported elsewhere (
5) that prepulse inhibition is lower in Vietnam veterans with PTSD than in civilians without PTSD but not lower than that in combat veterans who did not have PTSD. Butler et al. (
7) also found no differences in prepulse inhibition between Vietnam veterans with and without PTSD. Thus, in some studies prepulse inhibition deficits have been associated with combat exposure, not with PTSD per se. It is possible that exposure to intense sounds of gunfire during training and combat could lead to a subtle hearing impairment that could affect the efficiency of the prepulse in reducing the magnitude of startle. Subtle hearing impairment might not be detected by the routine audiologic screening used in psychophysiological investigations.
The present finding of a link between abnormal startle and PTSD is consistent with clinical observations. The results also add to previous knowledge by showing that abnormal startle reactivity (i.e., during darkness) can be found in combat veterans without PTSD, thus suggesting that combat experience has enduring effects. Such effects warrant further investigation.