Research in primary care has shown that enhancing acute-phase treatment improves short-term outcomes
(1–
3). We conducted two randomized controlled trials, the Collaborative Care intervention studies, to bolster acute-phase treatment of depressed patients in primary care
(1,
2). In these studies, primary care physicians and on-site mental health consultants collaborated to care for depressed patients. These multifaceted interventions resulted in better adherence and more intensive and appropriate pharmacotherapy as well as higher patient satisfaction than routine treatment in primary care. Among patients with minor depression, the intervention and comparison groups experienced equally favorable outcomes. However, patients with major depression showed significantly improved depression outcomes at 4 and 7 months after initiation of enhanced treatment.
This article reports the 19-month outcomes for the patients with major depression who participated in this acute-phase program. We tested whether patients who received the multifaceted interventions experienced better medication adherence and clinical outcomes than patients given routine treatment in the subsequent year.
METHOD
We have described in detail the methods of the Collaborative Care intervention trials in previous publications
(1,
2). Human subject committees approved the procedures, and all subjects gave written informed consent. Primary care physicians referred to the studies depressed patients who were willing to start antidepressant medicine. Exclusion criteria included age under 18 or above 80 years, mild depression (a depression score of less than 0.75 on the Hopkins Symptom Checklist [HSCL]
[4]), current alcohol abuse, psychotic symptoms, immediate suicide risk, dementia, pregnancy, terminal illness, and plans to disenroll from the health plan in the next 12 months. We randomly assigned 156 patients with major depression to receive either the enhanced acute-phase treatment or routine treatment. Of these patients, 116 (74.4%) completed the 19-month assessment: 63 patients in the enhanced-treatment group and 53 patients in the routine-treatment group.
The Collaborative Care programs included physician training, patient education, and reorganization of services (on-site mental health consultants were available to co-manage depressed patients with their primary care physicians, and there was prospective monitoring of medication adherence). All intervention visits occurred during the initial 3 months (acute-phase treatment); medication adherence monitoring was carried out in the continuation phase.
The 19-month assessment included the following measures: a telephone version of the Inventory for Depressive Symptomatology
(5), which evaluated depressive symptoms according to DSM-III-R criteria; the HSCL; antidepressant medication adherence; and chronic disease score (a measure of medical comorbidity)
(6). Patients were classified as adherent to pharmacotherapy if they reported taking antidepressant medication for at least 25 days in the past month (15 days if they were taking fluoxetine). Pharmacy refill records were examined to determine which patients used antidepressant medicines for at least 90 days at a dose that was in the range recommended by Agency for Health Care Policy and Research guidelines
(7,
8).
Clinical outcomes were compared statistically by using chi-square tests, t tests, and analyses of covariance controlling for age, sex, baseline HSCL or Inventory for Depressive Symptomatology score, and chronic disease score. To estimate the effects of dropping out of the study, baseline characteristics of patients who completed the 19-month assessments were compared with those of patients who did not.
RESULTS
The mean age of the 116 patients who were randomly assigned to the two treatment groups was 44.1 years (SD=13.6); 94 (81.0%) were women, 54 (46.6%) were married, and 90 (77.6%) had completed 1 or more years of college. At the baseline assessment, the patients’ mean HSCL score was 46.6 (SD=10.9) and their mean Inventory for Depressive Symptomatology score was 46.0 (SD=10.2). Seventy-six (65.6%) had had two or more previous episodes of major depression or had been experiencing chronic depressive symptoms for 2 years or more. Patients enrolled in the original Collaborative Care studies did not differ from patients who refused to do so in sex, age, or medical comorbidity
(1,
2). Furthermore, no significant differences existed at baseline between those who completed the 19-month follow-up interviews and the original group in sex, age, HSCL or Inventory for Depressive Symptomatology score, or percent receiving the intervention.
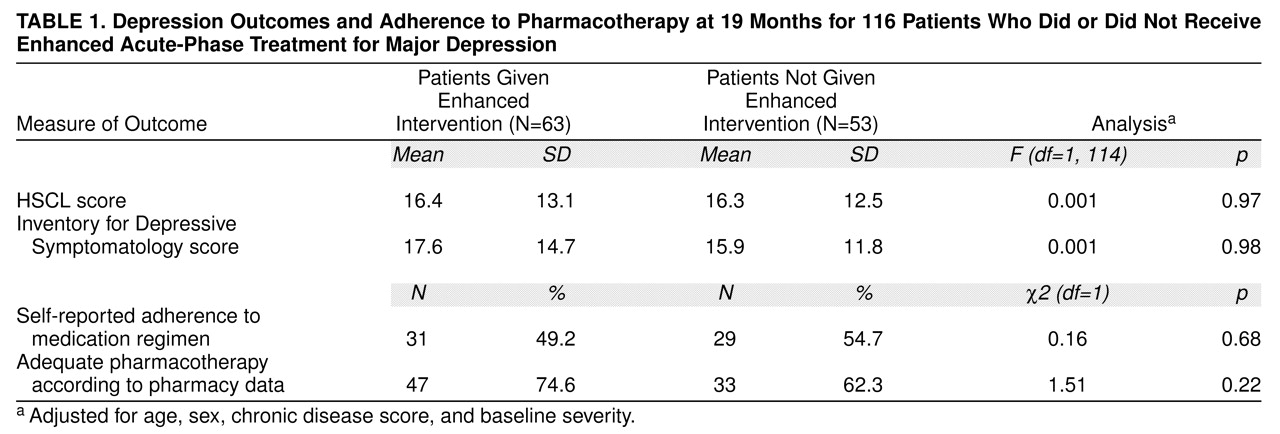
Table 1 shows that there was no significant difference in self-reported use of antidepressant medication between the enhanced intervention and comparison groups 19 months after random assignment to the two treatment groups. Automated pharmacy data substantiated the similar adequacy of pharmacotherapy in the two groups. In the year following the acute and early continuation phase, there was no difference in depression outcomes between the enhanced intervention and comparison groups as measured by HSCL or Inventory for Depressive Symptomatology scores (adjusted for age, sex, chronic disease score, and baseline HSCL or Inventory for Depressive Symptomatology score). Unadjusted analyses yielded similar results. Only three (4.8%) of the enhanced intervention patients and one (1.9%) of the comparison subjects met DSM-III-R criteria for major depression at the 19-month follow-up.
DISCUSSION
As reported in the Collaborative Care randomized trials
(1,
2), patients with major depression receiving enhanced acute-phase interventions achieved better short-term outcomes in terms of medication adherence and improvement in depression symptoms. However, these improvements did not endure. Intervention and comparison patients did not differ in medication use or severity of depressive symptoms in the subsequent year. In spite of a substantial rate of relapse (about 37%) reported in a related study
(9), few patients from either the enhanced intervention or the comparison group met criteria for major depression at the 19-month follow-up.
Generalizability of the findings of this study to the overall population of depressed primary care patients is limited because depressed patients who were not diagnosed as having depression by their physicians were not included in this study. Although there was a 26% loss to follow-up over the 19-month study period, patients included in this study group were similar at baseline to patients who were lost to follow-up.
The Collaborative Care programs bolstered interventions in the first 12 weeks of treatment by educating patients and physicians and by reorganizing primary care services. This enhanced and systematic management of depression ended early in the continuation phase of treatment, and routine care was then given. Our findings suggest that improved acute-phase treatment produced only short-term results. Although landmark specialty-based trials have demonstrated the benefit of longer treatment for patients at high risk of relapse
(10), these intensive models are probably not feasible in primary care. Further research is needed to test appropriate models for improving long-term outcomes for depressed patients treated in primary care.