The stereotypical behaviors observed in schizophrenia are thought to indicate reduced cognitive control of motor activity
(1) . Generating behavioral variety (novelty) requires such control and implicates the dorsolateral prefrontal cortex
(2) . When patients with schizophrenia are asked to deliberately vary the distribution of their activity in time, thereby exhibiting a temporal response space, activation of the left dorsolateral prefrontal cortex is positively correlated with the amount of variability that they generate. Those with greater left dorsolateral prefrontal cortex activation exhibit wider response space, which is indicative of better cognitive control
(3) .
In the present study, we aimed to investigate the therapeutic modulation of this activation/cognitive control relationship by combining concurrent functional magnetic resonance imaging (fMRI) with exposure to a putative cognitive enhancer: modafinil (2-[(diphenylmethyl) sulfinyl] acetamide)
(4,
5) . Modafinil has evinced cognitive enhancing effects in healthy subjects and people with schizophrenia
(4) . In people with schizophrenia, the effects of modafinil may be more pronounced in those with worse executive function
(5) .
Method
We studied 12 male subjects with DSM–IV schizophrenia and negative symptoms (mean age=37 years [SD=8]; Schedule for the Assessment of Negative Symptoms total score: mean=12 [SD=3]) who comprised a subset of a previously reported group
(5) without prominent positive symptoms (Schedule for the Assessment of Positive Symptoms total score: mean=4 [SD=2]). After complete description of the study, written informed consent was obtained from each subject. The study was approved by our local research ethics committees.
At initial assessment, each subject undertook a standard test of prefrontal executive function from the Multilingual Aphasia Examination: letter fluency, an executive task known to implicate the left prefrontal cortex. Subjects were then studied on 2 days, a week apart, in a double-blind crossover design. On each study day, subjects were randomly assigned to receive oral modafinil (100 mg) or placebo (and vice versa, following crossover) 2 hours prior to fMRI scanning.
Inside the scanner, subjects performed a task (the Sheffield Activity IN Time, or SAINT, task
[3] ) in which they were asked to generate sequences of motor activity by using their right index finger to press a button and were instructed to vary their responses in time, i.e., to avoid periodic (stereotypical) response patterns. Temporal variation in intrascanner motor activity is quantified by the coefficient of variation with respect to inter-response intervals for each scanning session. Better task performance (greater temporal variability, response space) is associated with larger coefficient of variation. For each subject, we calculated the difference in their coefficient of variation between the experimental conditions (modafinil minus placebo).
Images were acquired at 1.5 T and analyzed using statistical parametric mapping 99 (www.fil.ion.ucl.ac.uk) with protocols previously described
(3) . Each subject had two fMRI data sets (modafinil and placebo); these were used in a mixed-effects group analysis to produce a map showing brain areas more activated during the task for modafinil compared with placebo. For each subject, we also estimated intersession change in fMRI signal within the left dorsolateral prefrontal cortex clusters revealed by the group-level analysis to be more activated in the modafinil group than placebo condition.
Results
Compared with the placebo condition, administration of modafinil was associated with greater activation of the left dorsolateral prefrontal cortex (Brodmann’s area 9: t=2.42, df=11 [19 voxels exceeded threshold p<0.05, uncorrected]; Brodmann’s area 46: t=2.78, df=11 [54 suprathreshold voxels]) and right dorsolateral prefrontal cortex (Brodmann’s area 9: t=3.87, df=11 [86 suprathreshold voxels]; Brodmann’s area 46: t=2.96, df=11 [93 suprathreshold voxels]).
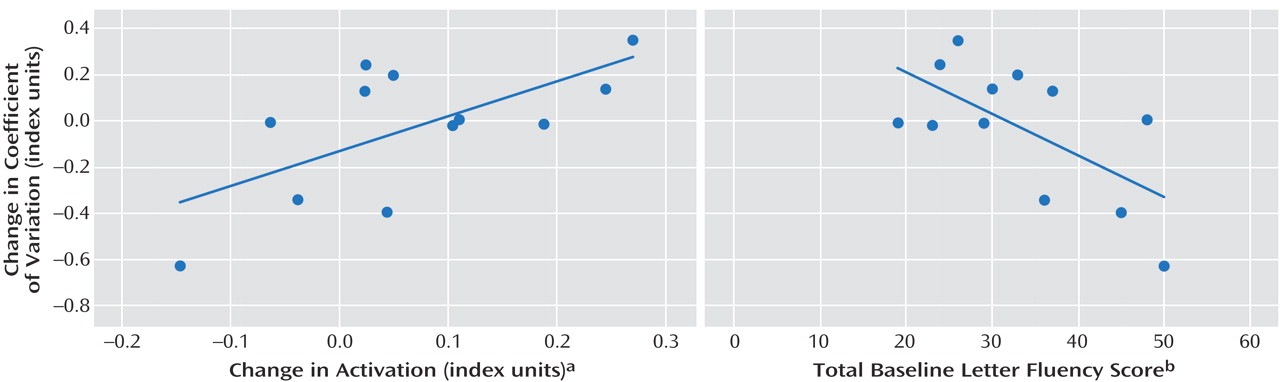
For the left Brodmann’s area 46, specifically, modafinil-induced change in activation exhibited a positive correlation with change in coefficient of variation during the task (r=0.65, p=0.02) (
Figure 1 ). The baseline letter fluency score was negatively correlated with change in coefficient of variation, suggesting a greater effect of modafinil in individuals with worse initial executive function (r= – 0.64, p=0.03) (
Figure 1 ). A priori linear regression with change in activation and the total baseline letter fluency score as predictor variables were able to account for most of the variance (adjusted r
2 =0.54) in the behavioral dependent variable, change in coefficient of variation (F=7.57, df=11, p=0.01) (
Figure 1 ).
Post hoc placebo coefficient of variation (another measure of baseline executive function) was negatively correlated with both change in activation (r=–0.71, p=0.01) and change in coefficient of variation (r=–0.62, p=0.03), supporting the notion that individuals with worse preexisting executive function exhibit greatest response to modafinil in terms of left dorsolateral prefrontal cortex (Brodmann’s area 46) activation
and behavioral task performance. Baseline letter fluency scores were also negatively correlated with change in activation, although this did not exceed the threshold for statistical significance, a finding compatible with the main regression analysis (see
Figure 1 ) because of the principle that very closely correlated predictor variables do not (taken together) explain more variance in the dependent variable than either taken in isolation (r=–0.32, p=0.32). Importantly, there was no negative correlation between baseline letter fluency scores and placebo coefficient of variation, excluding the possibility that those with better executive function failed to engage during the task (r=0.45).
The heterogeneous behavioral response to modafinil revealed by our regression analysis was also manifest, with no significant group-level difference in coefficient of variation between the modafinil and placebo conditions (t=0.35, df=11, p=0.73). Scanning order, i.e., modafinil received prior to or following crossover, was unrelated to whether behavioral performance increased or decreased (p>0.99, Fisher’s exact test).
Discussion
These data demonstrate that modafinil increases dorsolateral prefrontal cortex activation during the purposeful modulation of motor activity in time by people with schizophrenia. We have shown that where baseline prefrontal function is worse (as measured by letter fluency performance), increasing left dorsal lateral prefrontal cortex activation predicts improving cognitive control of motor activity (expanding the response space on a second executive task known to implicate left prefrontal cortex). This model is able to explain most of the variance (54%) in cognitive control that we observed. Clinically, these data suggest that modafinil is a cognitive restorer in some, improving cognitive control in relatively poor performers of these executive tasks. Furthermore, imaging and neuropsychological assessment may be helpful in characterizing those patients likely to derive benefit.
The absence of a group-level increase in cognitive control of motor activity was because of predictable intersubject variance in behavioral response to modafinil. It is clinically crucial to emphasize that some of the predictive power of our regression model arises because those subjects with better executive function were more liable to exhibit the minority (or “inverse”) response to modafinil, i.e., reduced activation of the left dorsal lateral prefrontal cortex and decreased cognitive control. Our data support the notion that modafinil is more likely to be associated with increased cognitive control where baseline executive function has been suboptimal.