A growing body of knowledge links psychopathology to functional brain alterations in anxiety disorders, such as posttraumatic stress disorder (PTSD)
(1–
3), specific phobia
(4–
6), panic disorder
(7–
9), and social phobia
(10–
12). Through neuroimaging, the orbitofrontal, prefrontal, insular, temporal, cingulate, parietal, and occipital cortices have been identified as important neural network nodes in pathological anxiety. Furthermore, there is a convergence of findings from lesion
(13–
15), animal
(16), and neuroimaging
(2,
11,
17–
19) studies demonstrating that the amygdaloid complex plays a crucial role in the perception and production of fear. However, the neural correlates of anxiety provocation in patients with social phobia, arguably the most common anxiety disorder
(20), have been studied to a limited extent with preliminary data only
(10–
12). Therefore, the aim of the present study was to examine the functional neuroanatomy of the provocation of social anxiety in subjects with social phobia during a public speaking task, using the same verbal task without an audience for baseline measures. To explore the neural pattern that is specific for social phobia, we included a healthy nonphobic comparison group.
There are several studies of the redistribution of regional cerebral blood flow (rCBF) resulting from perceptually induced anxiety in patients with specific phobia
(4,
5,
21,
22), social phobia
(11–
12), and PTSD
(1,
19). We reviewed those studies to establish a priori hypotheses concerning which brain territories to target. During anxiety provocation, increased rCBF has often been observed in the secondary visual cortex
(1,
4,
5,
22), except in subjects with social phobia, in whom reductions were observed
(12). Because the present study involved anxiety provocation in subjects with social phobia, we predicted involvement of the secondary visual cortex, but we did not predict the direction of change. Enhanced amygdaloid blood flow has been reported in subjects with social phobia during aversive conditions
(17,
23) and in normal subjects during fear conditioning
(24–
26). Since social phobia in part reflects negative learning contingencies related to fear conditioning
(27), we predicted increased blood flow in the amygdaloid complex during stressful tasks.
The posterior cingulate cortex, particularly the retrosplenial area, and the inferior frontal cortex have consistently been associated with increased perfusion during normal emotional experiences
(28). In individuals with animal phobia, rCBF is down-regulated in the posterior cingulate cortex as a function of anxiety provocation
(5,
22), whereas increased posterior cingulate activation has been observed in subjects with social phobia
(11) and trauma patients
(1). Regional CBF in the anterior cingulate cortex was found to be altered in patients with PTSD (19) and social phobia
(11). However, these changes did not have a uniform direction, since both decreased
(19) and increased
(11) activity have been reported. Thus, we predicted involvement of the posterior and anterior cingulate cortices during symptom provocation but not whether rCBF would increase or decrease.
Frontal activity, including in the prefrontal and orbitofrontal areas, seems attenuated during symptom provocation in most
(1,
4,
5,
12,
21,
22) but not all
(11,
19) studies of anxiety provocation. A similar pattern is evident for neural alterations in the temporal cortex, since some
(1,
4,
5,
22) but not all
(11) studies report lower rather than higher rCBF while subjects experience fear. Hence, we predicted lower activity in the frontal and temporal cortices while the subjects were experiencing social anxiety. Finally, we predicted decreased activity in the insular and parietal cortices because the former seems to be involved in a negative feedback loop controlling autonomic nervous system activity while subjects are experiencing aversive conditions
(29). Decreased parietal rCBF was also reported by Bremner and colleagues
(1) during provocation of posttraumatic anxiety.
In summary, brain areas that have previously been reported to alter neural activity as a consequence of anxiety provocation were investigated in individuals with and without social phobia during public (i.e., speaking in front of an audience) and private (i.e., speaking alone) speaking.
Discussion
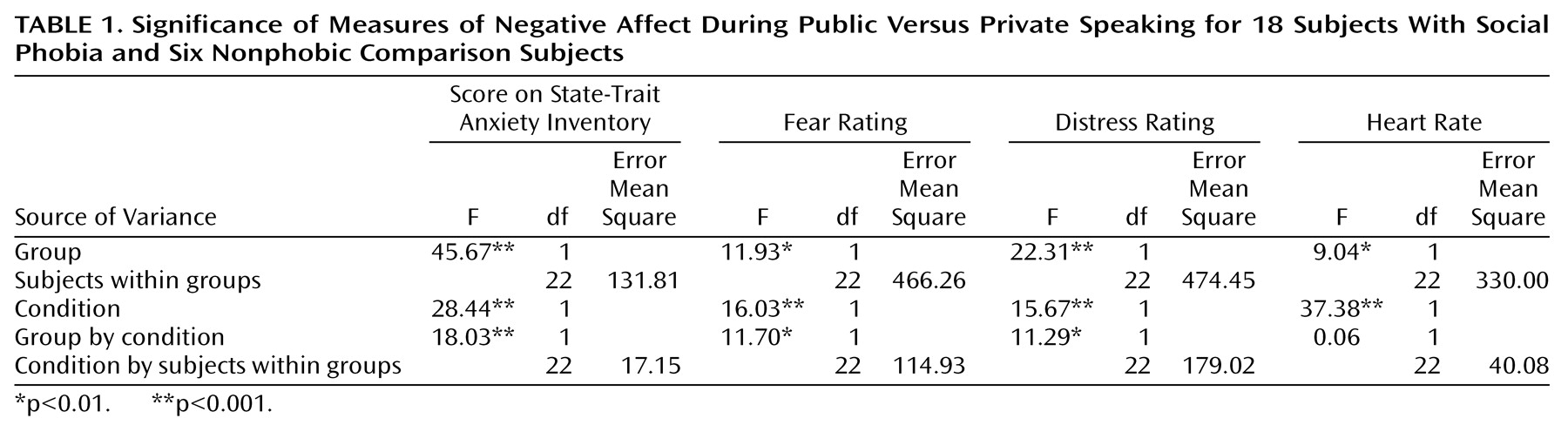
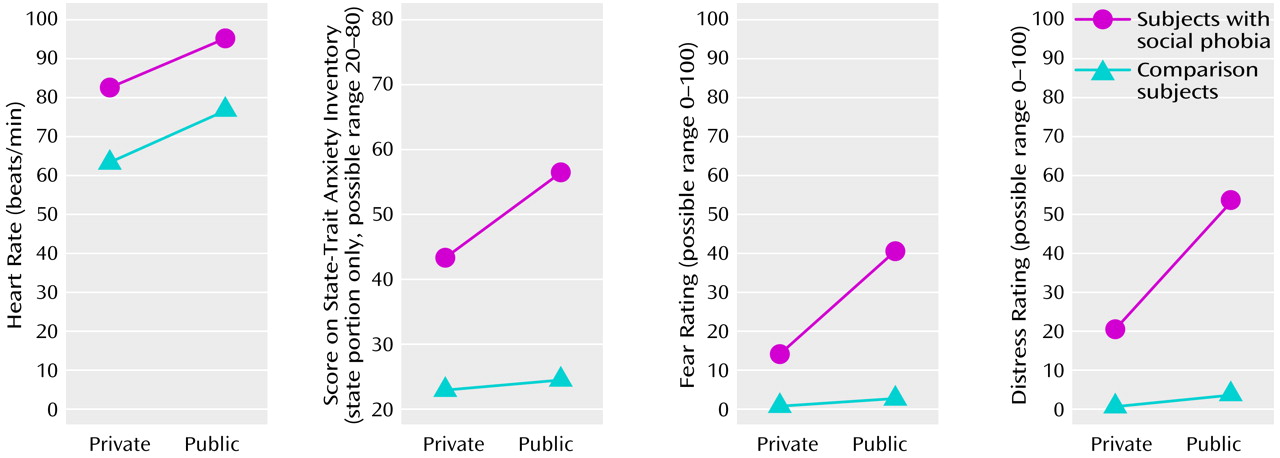
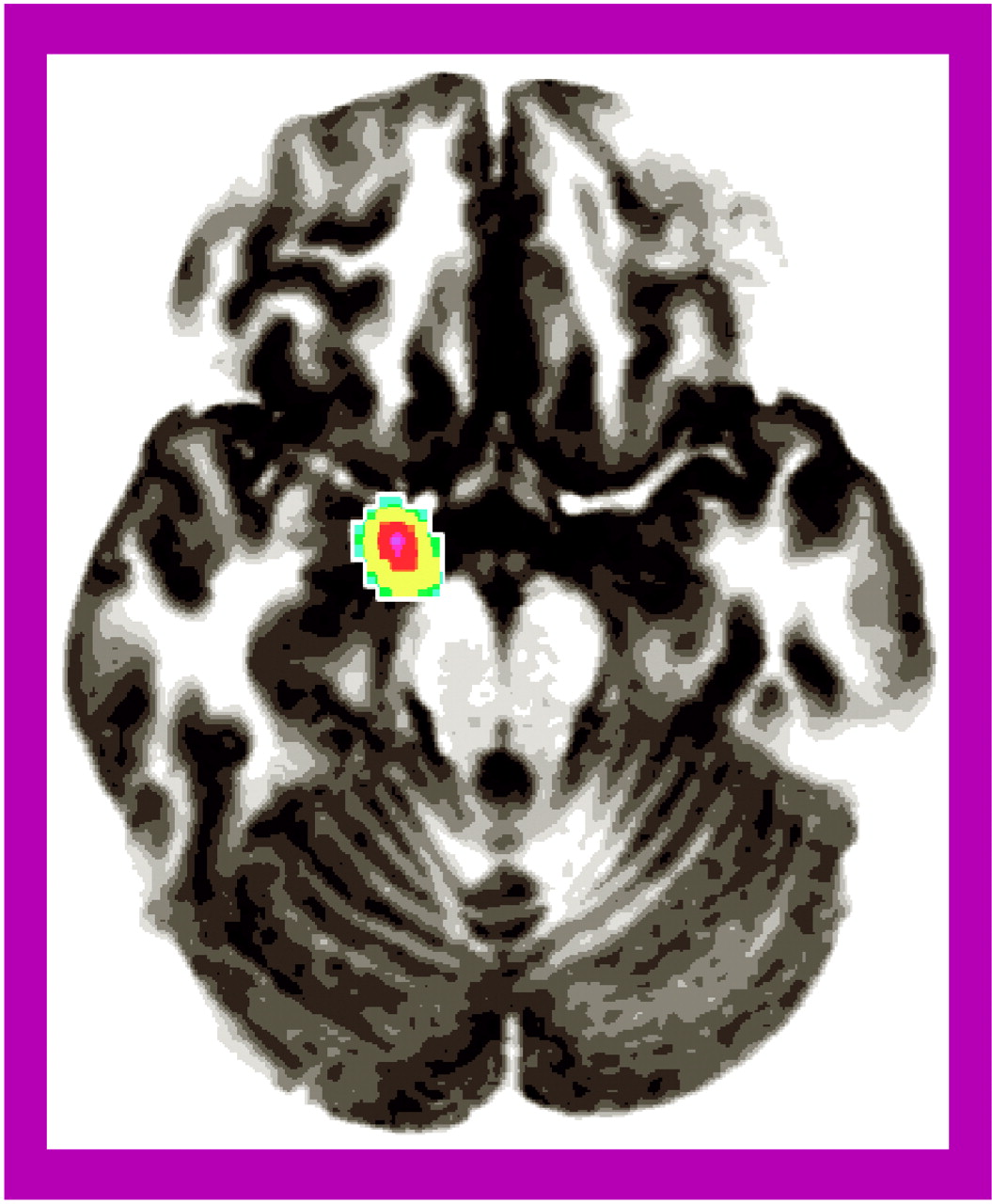
Our aim was to explore the functional neuroanatomy of social phobia by recording rCBF in subjects with social phobia during public and private speaking compared to that in a healthy nonphobic comparison group. The subjects with social phobia spoke in public while experiencing more anxiety than the nonphobic subjects, whereas anxiety differences were smaller during private speaking. Increased fear and anxiety was associated with increased rCBF in the right amygdaloid complex, which is consistent with the findings from previous studies identifying the amygdaloid complex as important for emotion, particularly fear (for a review, see reference
44). The right lateralized activations are in line with theories of emotion that emphasize the right hemisphere as being dominant in experiencing negative affect
(45). Furthermore, aversively controlled behavior induces increased long-term potentiation of efferents of the right amygdala in animals
(46). Cortically, brain blood flow decreased in the social phobics and increased in the comparison subjects more during public than private speaking in the orbitofrontal and insular cortices as well as in the temporal pole and increased less in the social phobia group than in the comparison group in the parietal and secondary visual cortices. Furthermore, rCBF increased in the comparison group, but not in the social phobics, in the perirhinal and retrosplenial cortices. This neural pattern most likely reflects emotional processes, since the public and private speaking tasks were identical for both groups and were associated with an anxious reaction during public speaking in the subjects with social phobia but not in the nonphobic subjects. Because we evaluated the interaction between the subjects with social phobia and the nonphobic comparison subjects and public versus private performance, rCBF alterations resulting from perceptual or anatomical differences can be ruled out. Instead, they seem to reflect the emotional impact of the situation on the subjects.
Consistent with other PET studies of symptom provocation in subjects with anxiety disorders
(2,
11,
19), we observed increased rCBF in the amygdaloid complex during anxiety provocation, which may be consistent with Reiman’s description
(11) of a localized alarm center in the amygdala and hippocampus. Hence, social anxiety seems associated with increased activity both in the amygdala and hippocampus. Birbaumer et al.
(17) also observed that the amygdala was activated in the processing of neutral faces in subjects with social phobia but not in nonphobic subjects. A complementary explanation for the rCBF activations in the amygdaloid complex could be that evaluation of ambiguous cues
(47), such as neutral faces, is supported by the amygdala in subjects with social phobia, whereas cortical areas perform this evaluative process in nonfearful subjects. Thus, there is a fear-related shift from cortical to subcortical processing in subjects with social phobia when encountering situations causing symptom activation.
Individuals with phobic disorders are characterized by an inability to control fear in phobic situations
(48). Hence, the rCBF reductions in the orbitofrontal cortex (Brodmann’s area 12), together with enhanced rCBF in the amygdaloid complex, may be associated with emotional dysregulation linked with failure to inhibit negative affect
(49). For example, Morgan and co-workers
(50) demonstrated that lesions to the medial prefrontal cortex prolonged fear extinction. The orbitofrontal cortex seems particularly involved in voluntary emotional control
(5), and lowered orbitofrontal perfusion has also been observed during anxiety provocation in subjects with specific phobia
(5,
21,
22), PTSD
(51), and panic attacks
(52). Because a reciprocal relation between rCBF in the subgenual cingulate and the prefrontal cortex has been described in depression and normal sadness
(53), we could speculate that negative affect in general is associated with limbic-cortical reciprocity.
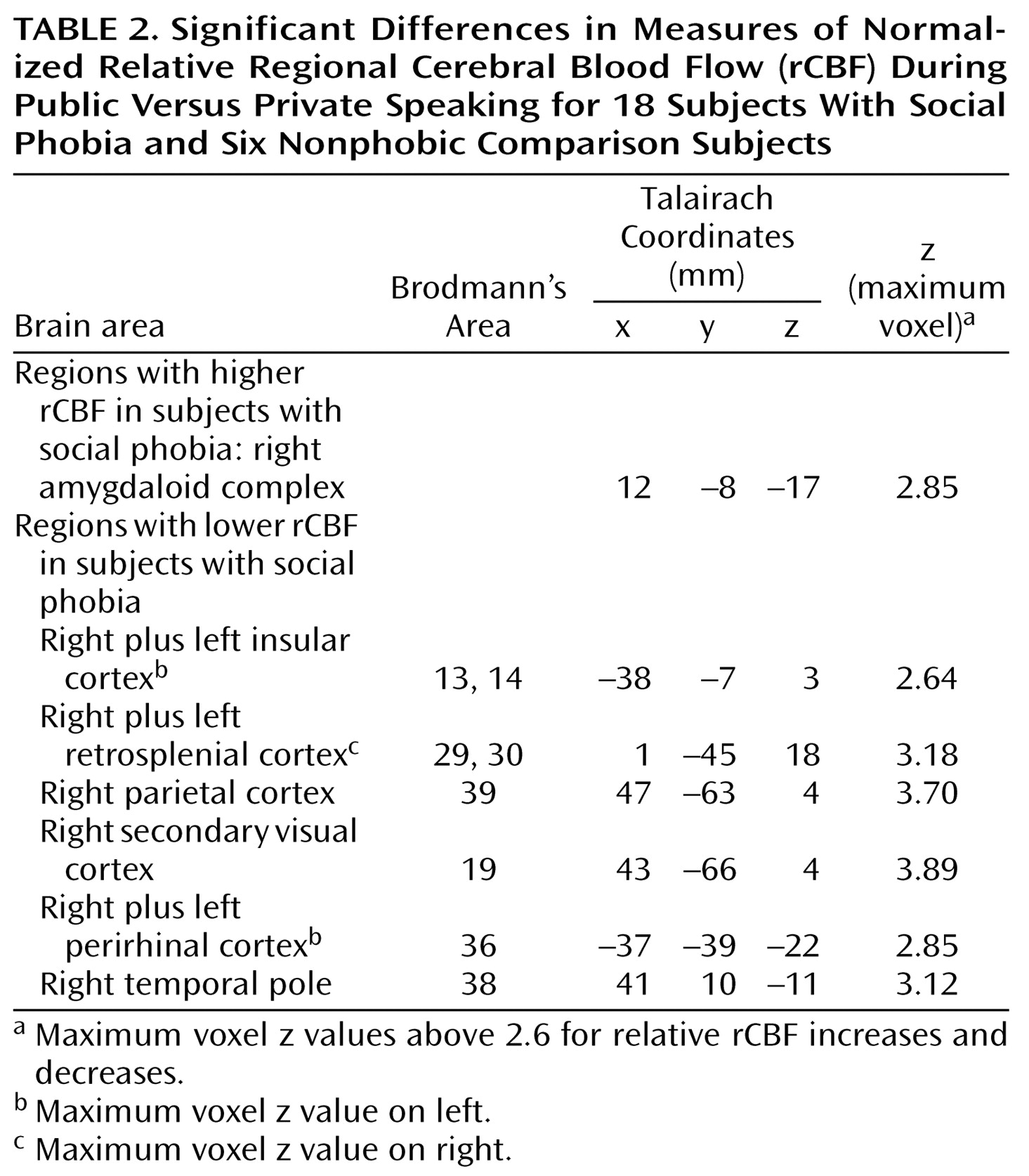
Public speaking anxiety in subjects with social phobia seems associated with altered neural activity in subcortical and cortical structures of relevance for affect and attention. The neural alterations in the present study, located in the secondary visual (Brodmann’s area 19), retrosplenial (Brodmann’s areas 29 and 30), parietal (Brodmann’s area 39), and temporal (Brodmann’s areas 36 and 38) cortices, have previously been suggested to be involved in emotional evaluative processes (4, 11, 28, 54, 55), e.g., visuospatial processing
(56). However, perception of novel faces in nonphobic subjects activates a network that includes the right parietal and secondary visual cortices, as well as the right fusiform gyrus
(57,
58). Thus, it is likely that the relatively higher neural activity in nonphobic subjects than in subjects with social phobia indicates cortical rather than subcortical evaluative processes. The reverse characterizes subjects with social phobia, and amygdala activation is associated both with perception and generation of emotion (for a review, see reference
44).
Altered rCBF in the insular cortex (Brodmann’s areas 13 and 14) could reflect regulation of emotional autonomic responses
(29,
44). For example, we recently reported a negative correlation between insular blood flow and measures of activation in the autonomic nervous system, which suggests a negative feedback function for the insula
(29). The pattern of change in the present study also indicates an inverse relation between autonomic anxiety indices and insular perfusion.
In previous PET studies, social anxiety has been associated with 1) enhanced perfusion in the right dorsolateral prefrontal and the left parietal cortices
(10), 2) deactivations in the visual and medial frontal cortices
(12), and 3) rCBF increases in the lateral prefrontal, sensorimotor, anterior temporal, and midcingulate cortices as well as in the thalamus. Increases that approached significance were reported for the amygdala, hippocampus, hypothalamus, and cerebellar vermis as well as for the anterior cingulate and medial prefrontal cortices
(11). Because these studies
(10–
12) reported only preliminary data and since the completed studies do not seem to have been published, it is difficult to properly evaluate and compare these results with ours.
Traditionally, z scores of 3.09 that are sometimes corrected for multiple comparisons
(1) and sometimes not
(19) are judged significant. In the present study, areas in which the direction of change was predicted produced z scores that varied from 2.64 to 3.70, whereas areas in which we did not predict the direction of change, only involvement, had z scores exceeding 3.09. A z score of 2.58 corresponds to a one-tailed probability of 0.005 and a score of 3.09 to a two-tailed probability of 0.002. Because rCBF alterations in all areas studied were theoretically predicted on the basis of previous independent research, we believe that they are unlikely to reflect statistical type I errors. Of course, independent replication of the present data would strongly support our findings and interpretations. Although these data are preliminary and were compared with those from a small nonphobic group, the results are consistent with a wealth of data showing that alterations in the amygdala are associated with fear and anxiety
(44).
Recently, Paradiso and co-workers
(59) reported that observing and assigning emotional value to unpleasant stimuli activated subcortical limbic regions, whereas evaluation of pleasant stimuli activated cortical paralimbic areas. They suggested that the former reflects activity in an archaic danger-recognition system and the latter, a phylogenetically younger system. To conclude, we speculate that subcortical activations observed in the subjects with social phobia during symptom provocation represented anxiety-related activation of this phylogenetically older danger system, whereas cortical activity in the nonphobic subjects represented evaluative activity in a phylogenetically younger system.