Elevated homocysteine levels in plasma are considered a risk factor for cardiovascular and cerebrovascular disease
(1). Recently, elevated plasma homocysteine has also been found to be a risk factor for Alzheimer’s disease
(2), suggesting that some risk factors can accelerate or increase the severity of several CNS disease processes. Similarly, apolipoprotein E-4, originally found to be a risk factor for Alzheimer’s disease, is apparently also a risk factor for vascular dementia and exacerbates the severity of other CNS degenerative disorders.
Kruman et al.
(3) reported that homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. An oral methionine load has classically been reported to exacerbate schizophrenia and is of course converted to homocysteine
(4). Regland et al.
(5) reported a case of a young patient with schizophrenia with a significantly elevated serum level of homocysteine. She improved repeatedly after cobalamin (B12) injections and deteriorated during periods without treatment. Methylenetetrahydrofolate reductase activity in cultured skin fibroblasts of this patient was reduced to a magnitude that is found among people with heterozygous deficiency. A defect in the reductase activity causes a deficiency in methylenetetrahydrofolate, with a consequent reduction of the remethylation of homocysteine to methionine.
The gene for methylenetetrahydrofolate reductase is polymorphic in the human population. In its homozygous form, a C677T mutation occurs in more than 5% of the population and produces a thermolabile variant that reduces the overall enzyme activity to less than 30% of normal
(6). Several small studies and case reports suggested that homozygosity for the T677 allele of the methylenetetrahydrofolate reductase gene—encoding for the thermolabile enzyme associated with hyperhomocystinemia—may be associated with higher rates of schizophrenia.
Regland et al.
(7) studied 11 patients with schizophrenia-like psychosis. Seven of the patients, six men and one woman, were homozygous for thermolabile methylenetetrahydrofolate reductase. In the patients who were homozygous for the C677T mutation, the homocysteine concentrations did not respond to vitamin B12 but were normalized by folate supplementation. In the patients with homozygotes for the normal alleles, however, the homocysteine concentrations were reduced by vitamin B12 alone. Regland et al. suggested that homozygosity for thermolabile methylenetetrahydrofolate reductase is a risk factor for schizophrenia-like psychosis and that this risk may be reduced by folate supplementation.
Susser et al.
(8) conducted a pilot study comparing homocysteine levels of patients with schizophrenia and normal comparison subjects with and without low folate levels. In the low folate group (six patients, eight comparison subjects), mean homocysteine was 10.7 μM in patients with schizophrenia compared with 7.7 μM in comparison subjects (p=0.03), whereas in the non-low-folate group (11 patients, 16 comparison subjects), mean homocysteine did not differ between patients with schizophrenia and comparison subjects. These pilot data are compatible with the hypothesis that a folate-sensitive defect in homocysteine metabolism contributes to some cases of schizophrenia.
We screened plasma homocysteine levels in patients with schizophrenia compared with a group of normal subjects.
Method
The study was approved by the Helsinki Committee (institutional review board) of Ben Gurion University for the patients with schizophrenia and the Helsinki Committee of Sheba Medical Center for the comparison subjects. All subjects provided written informed consent.
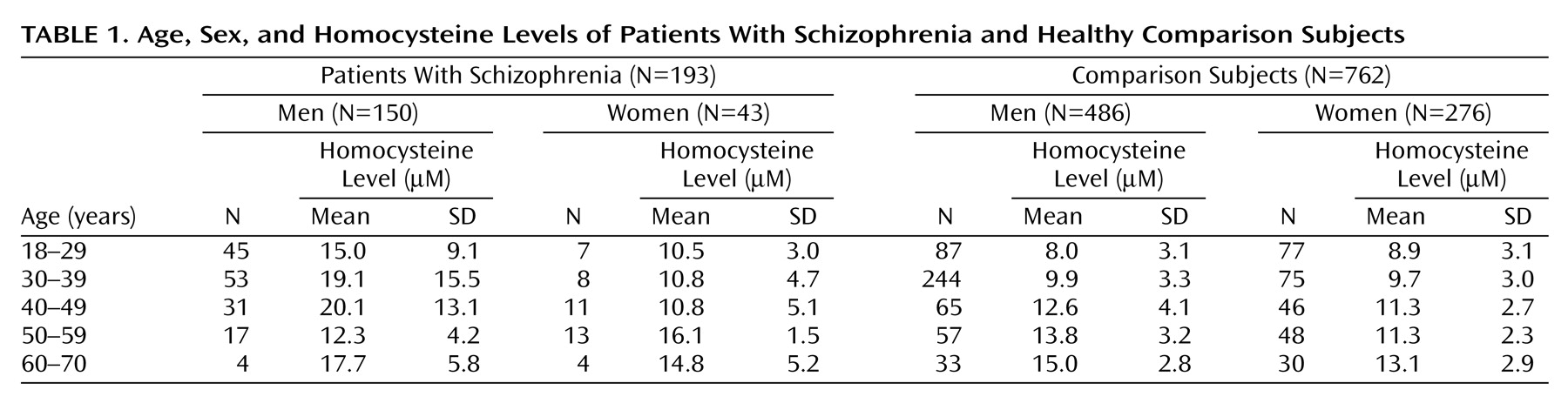
The patient group was composed of 193 male (N=150) and female (N=43) patients with chronic schizophrenia diagnosed according to DSM-IV. The patients’ age range was 18–70 years, and none had a history of neurological or cardiovascular disorders or drug or alcohol abuse. All patients were being treated with antipsychotic medications and were in a variety of clinical settings, including acute inpatient units, chronic inpatient units, hostel care, and outpatient care.
Comparison subjects were 762 men (N=486) and women (N=276) whose plasma homocysteine levels were determined at the Sheba Medical Center Institute of Clinical Pathology as part of employee health screening. These subjects were 18–70 years old, and the homocysteine determinations in their blood samples were run in the laboratory over the same time period as the samples from patients with schizophrenia.
Fasting morning blood samples were obtained in ice-cooled EDTA tubes. Plasma was separated by centrifugation at 5°C and stored at –20°C. Total homocysteine levels were measured by high-performance liquid chromatography technology with fluorescence detection following labeling of homocysteine with monobromobimane according to a modification of the method of Araki and Sako
(9).
Results
Table 1 presents the homocysteine levels of patients and comparison subjects. A one-way analysis of covariance with age and sex as covariates was performed. The effect of schizophrenia was marked (F=135.7, df=1, 951, p<0.0001): the mean homocysteine level was 16.4 μM (SD=11.8) in patients with schizophrenia, compared with 10.6 μM (SD=3.6) in healthy comparison subjects (the covariance-adjusted means were 16.3 μM for patients and 10.6 μM for comparison subjects). The significant difference was entirely in male patients with schizophrenia who were younger than age 50.
Discussion
To our knowledge, this is the first comprehensive study of homocysteine levels in schizophrenia to include a range of age groups. Virgos et al.
(10) found plasma homocysteine levels no different in schizophrenic inpatients (N=210) and comparison subjects (N=218). However, the average age of the patients with schizophrenia and the comparison subjects was over 60, and in our study there was no difference between subjects in this age group.
Higher homocysteine levels in young male patients with schizophrenia could be related to the pathophysiology of aspects of this illness. For instance, it is known that onset of schizophrenia is earlier in male than female patients and that the illness more often has a chronic deteriorating course in young men. Homocysteine has been shown to be neurotoxic
(11), and it has been shown that stress can open the blood-brain barrier to some neurotoxic substances
(12). It is possible that the stress of acute psychosis allows high homocysteine levels to enter the brain and cause neurodegeneration, clinical deterioration, and chronicity.
This hypothesis does not make any assumption as to the origin of high homocysteine in young men with schizophrenia. It could be caused by smoking, lack of exercise, or poor diet. However, high homocysteine levels could negatively affect the course of illness through a biochemical mechanism. In Alzheimer’s disease, biochemical and genetic studies clearly point to β-amyloid and its metabolism as pathogenetic factors. However, plasma homocysteine levels could explain 16% of the attributable risk in Alzheimer’s disease
(2), demonstrating the powerfully multifactorial nature of most common diseases.
Several homocysteine-reducing strategies have been discovered, first for metabolic hyperhomocystinemia disorders
(13) and more recently in studies for reduction of risk from cardiovascular and cerebrovascular disorder
(14). Folic acid, cobalamin, and pyridoxine supplementation can markedly lower plasma homocysteine. It is conceivable that daily supplementation with these vitamins could prevent clinical deterioration in some patients with schizophrenia.