Gilles de la Tourette’s syndrome is characterized by multiple motor and phonic tics that wax and wane over the course of a person’s illness. DSM-IV criteria for Tourette’s disorder specify that the tics 1) occur many times a day (usually in bouts) nearly every day or intermittently for more than 1 year; 2) cause marked distress or significant impairment in social, occupational, or other areas of functioning; and 3) emerge before 18 years of age. The criteria established by the Tourette Syndrome Classification Study Group
(1) also require the presence of both motor and phonic tics. However, no impairment criterion is required. Both sets of criteria regard Tourette’s disorder to be a unitary disorder.
The diagnosis of Tourette’s disorder is relatively straightforward. However, there is considerable clinical variability across patients, and the degree to which other behaviors may be associated with the syndrome is not clear. For example, if Tourette Syndrome Classification Study Group criteria are used, an individual can receive a diagnosis by having three tics (two motor and one phonic) that have persisted over 1 year’s time. In contrast, another individual may exhibit a severe range of tics that encompasses many different types of behaviors ranging from simple eye-blinking tics to coprolalia and elaborate and complex compulsion-like behaviors. In fact, two individuals could have entirely different and nonoverlapping symptoms yet still fulfill diagnostic criteria for Tourette’s disorder.
There have been few other attempts to formally classify Tourette’s disorder patients on the basis of their tic phenotypes
(2,
3). On the other hand, because a number of investigators have observed that symptoms of other conditions (e.g., attention deficit hyperactivity disorder [ADHD], obsessive-compulsive disorder [OCD]) very often co-occur with Tourette’s disorder, there has been considerable work done to attempt to elucidate the phenotypic relationship between these conditions and Tourette’s disorder. For example, Jagger et al.
(4) reported that inattention and hyperactivity were among the first symptoms observed in a large percentage of Tourette’s disorder clinic patients. Comings and Comings
(5) proposed that ADHD represented a variant expression of the etiologic factors responsible for the manifestation of Tourette’s disorder and chronic multiple motor or phonic tics. Findings from our work
(6,
7) did not fully support this hypothesis. Data from our family study
(7) suggested that while there may be some etiological relationship between some forms of ADHD and Tourette’s disorder, it is unlikely that ADHD in the absence of tics is a variant expression of the genetic factors important for Tourette’s disorder. It is possible that ADHD is associated with increasing clinical severity but not with the underlying genetic diathesis. Any potential genetic relationship between ADHD and Tourette’s disorder continues to be an active area of investigation, and both disorders appear to be genetically complex.
Obsessive-compulsive behaviors have also been reported to be associated with Tourette’s disorder. In fact, Gilles de la Tourette anecdotally reported the obsessive-compulsive symptoms of one of his patients
(8). Subsequently, a number of investigators have reported higher than expected frequencies of OCD among Tourette’s disorder patients
(4,
9–
12). Family study data
(13–
16) suggest an etiological relationship between some forms of OCD and Tourette’s disorder.
While the elucidation of the relationship of Tourette’s disorder to other conditions is important, the current study was undertaken to determine if unique components could be identified within the symptom spectrum seen in patients with Tourette’s disorder. Component phenotypes may prove useful in the search for etiologic genes in Tourette’s disorder. Such an approach was used by Baer
(17) to better understand the complexity of OCD and yielded evidence for at least three unique components, while Leckman et al.
(18) found evidence for four components of the OCD phenotype. Analyses incorporating symptom components recently contributed to success in finding separate genetic loci contributing to reading disabilities
(19). Factor and cluster analyses for other psychiatric phenotypes, such as mania/bipolar disorder
(20) and autism
(21), have also generated new clinical interpretations and revealed previously undescribed heterogeneities. We conducted a principal-component factor analysis to provide a structural definition of the symptom dimensions of a Tourette’s disorder family data set.
Method
Patients
Tic symptom information was elicited from 85 Tourette’s disorder probands and their 28 affected first-degree relatives who participated in a previous family study of Tourette’s disorder. The total family study data set consisted of 86 probands and 338 first-degree relatives and has been described in detail elsewhere
(13). For this study, applicable symptom data were available on 85 probands; recurrence risks were calculated for their 331 first-degree relatives. Briefly, probands who met DSM-III-R criteria for Tourette’s disorder were randomly ascertained from the membership of the Tourette Syndrome Association. Because there were comparatively few female members, a greater percentage of them were invited to participate to ensure that a sufficient number of families of female probands would be available for data analyses.
Symptom Assessment and Diagnoses
After obtaining informed consent, all adult probands and available first-degree relatives were directly interviewed. Lifetime data for 29 motor and phonic tic symptoms were obtained by using a structured interview (the Schedule for Tourette and Other Behavioral Syndromes
[22]). The reliability and validity of this interview has been established, and it has been used successfully at our center for over a decade. Each symptom was scored as ever present or never present. All interviews were performed by bachelor’s degree-level interviewers trained to reliability. Additionally, family history information was solicited from participants about each of their adult relatives; this information was collected with a semistructured interview. Thus, two types of information about all participating probands and most relatives were available to the diagnostic raters. For each noninterviewed adult relative (22% of all relatives), two family history reports were obtained on average. Best-estimate diagnoses for relatives were made independently by two experienced raters; in case of diagnostic disagreement, consensus diagnoses were reached by following established procedures developed for other neuropsychiatric disorders
(23). For the exploratory factor analyses, only symptom data from the 85 probands were included.
Statistical Analysis
All analyses were performed by using SPSS
(24). Principal-component factor analyses are typically based upon nondichotomous variables. However, the use of dichotomous variables can be justified in exploratory approaches such as that reported here. A preliminary cluster analysis was performed to determine if any dichotomous symptoms could be combined into nondichotomous variables.
Previous reports of factor and cluster analyses for neuropsychiatric disorders have relied on an a priori clustering of dichotomous symptoms based upon clinically justified groupings
(15,
17,
18,
20,
21,
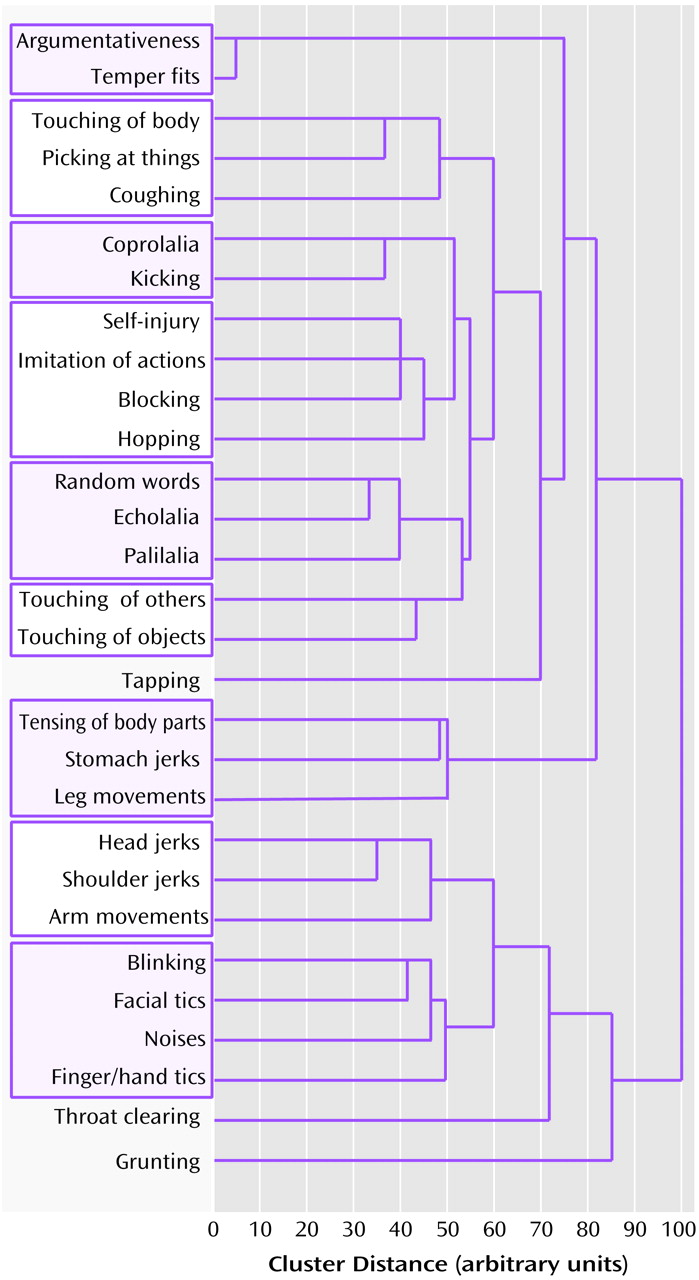
25). We instead performed an initial data reduction on the entire array of symptom variables for probands only, making no prior assumptions, by an agglomerative hierarchical cluster analysis (
Figure 1). This method progressively forms clusters of variables until all variables are subsumed into a single cluster; the average linkage between groups is used to evaluate the Euclidean-squared cluster distances. The stages of agglomeration were displayed as a dendrogram, with the formation of clusters at each stage plotted along a scaled, between-stage distance axis. For the current study, the best set of clusters required to adequately represent the data was determined by inspection of the dendrogram. Variables were classified as a cluster when 1) their dendrogram lines converged within a 10-unit window on the dendrogram cluster distance axis, and 2) the convergence occurred before 50 (a distance of 0 units represented the independent symptoms; 100 units represented the unitary cluster of all symptoms). For each resulting cluster, a score was then generated for each subject equal to the sum of the symptom variables contained therein: symptom variables were scored 1 for never present and 2 for ever present.
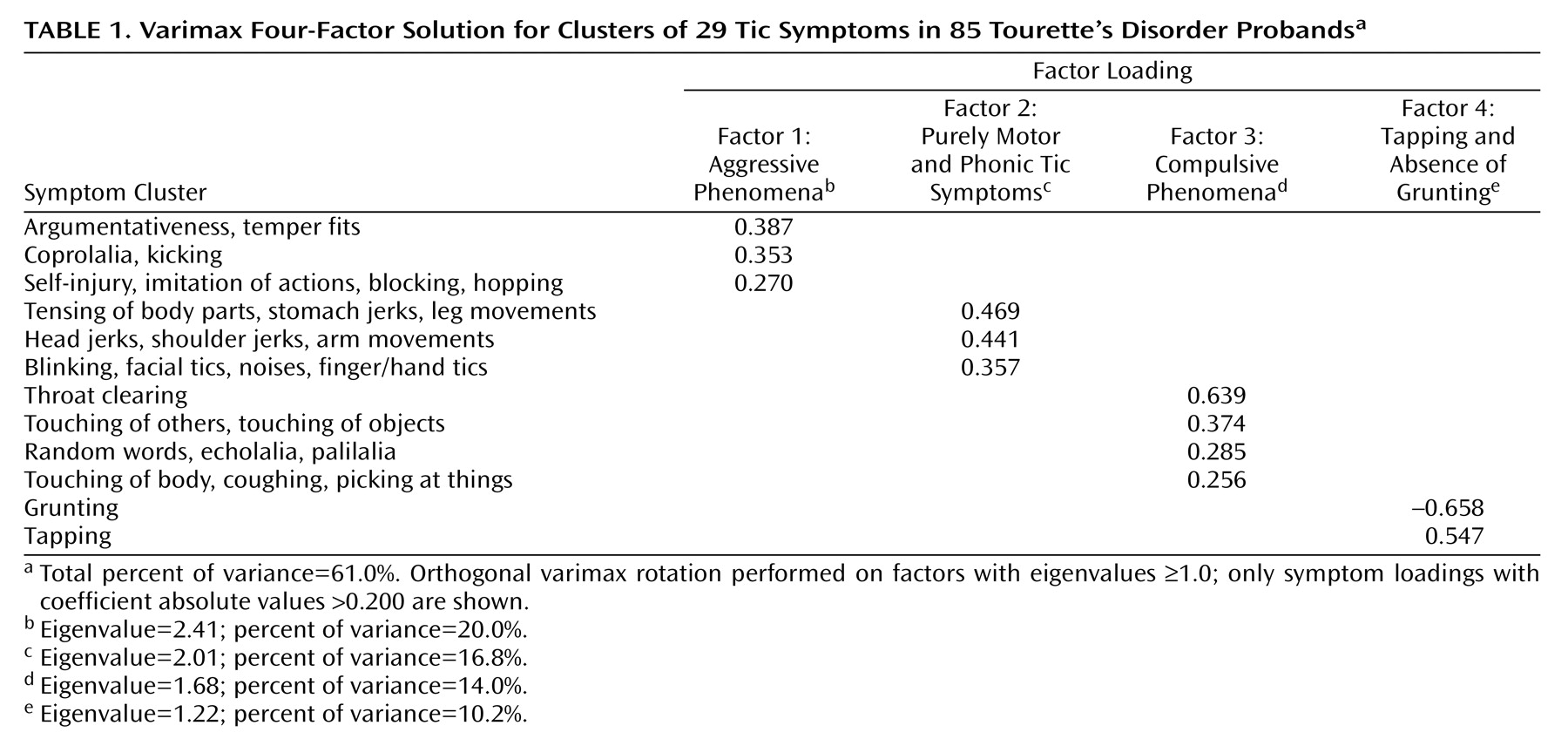
Scores for clusters resulting from the hierarchical analysis of proband symptoms were then used as input variables for the principal-component factor analysis. After factor extraction, an orthogonal varimax rotation was performed on factors with eigenvalues ≥1.0. This procedure minimized the number of variables with high loadings on each extracted factor and allowed for a more straightforward interpretation; other rotations were not explored. Symptom loadings with coefficient absolute values >0.200 were used to describe the factors.
Recurrence risks for OCD, ADHD, Tourette’s disorder, and chronic multiple tics among relatives of probands with high factor scores (>0) and low factor scores (≤0) were compared by using Fisher’s exact test. In order to determine if factor scores were important predictors of individual diagnoses, logistic multiple regressions were performed with the disease status as the dependent variable. Similar analyses examining the effects of factor scores on age at Tourette’s disorder onset were performed by linear regression with age at onset as the dependent variable. For the linear regressions, two-tailed Student’s t tests were used to assess significance; for logistic regressions, the Wald statistic was used. Within-family correlations between proband factor scores and affected relative factor scores were calculated by using intraclass correlations; F tests were used to assess significance. In order to increase available power, the intraclass correlation analysis included relatives affected with chronic multiple tics.
Results
The agglomerative hierarchical cluster analysis of the 29 tic symptoms for the 85 probands identified 12 symptom clusters. The dendrogram illustrating the progression of cluster formation and the final resulting clusters is shown in
Figure 1.
For each cluster, a score was generated equal to the sum of the elemental symptom variables. These scores were then used in the principal-component factor analysis. After factor extraction, an orthogonal varimax rotation was performed that minimized the number of variables having high loadings on a particular factor. Four factors resulted from the analysis, accounting for 61% of the symptomatic variance (
Table 1). Factor 1, which accounted for 20% of the variance, was characterized by aggressive behaviors that included being argumentative, having temper fits, self-injurious behavior, and coprolalia. Factor 2, which accounted for 17% of the variance, was characterized primarily by simple tics (both motor and phonic). Factor 3, which accounted for 14% of the variance, was characterized by compulsive behaviors such as throat clearing and repetitive actions (including touching of others or objects and repeating speech sounds). Finally, factor 4, which accounted for 10% of the variance, was characterized by the absence of grunting tics and the presence of tapping (which was distinguished from finger and hand tics or touching of others or objects).
To assess the external validity of the factors, we tested whether any were related to proband characteristics that were not used in the cluster or factor analyses (e.g., nonsymptom items such as age at onset and comorbidity of other conditions) or to recurrence risks for Tourette’s disorder, chronic multiple tics, OCD, and ADHD among first-degree relatives. Regression analyses indicated that factor 3 (compulsive phenomena) was associated with age at onset of Tourette’s disorder (F=5.54, df=1, 116, p=0.02); a higher score on this factor was associated with earlier onset. Furthermore, the occurrence of ADHD in probands with Tourette’s disorder was associated with factor 1 (aggressive phenomena; Wald χ2=12.20, df=1, p=0.0005) and factor 3 (Wald χ2=5.08, df=1, p<0.03). The association for both was in the expected direction: high factor scores were associated with the presence of ADHD. And finally, factor 2 (simple motor and phonic tics) was associated with the sex of the individual (Wald χ2=6.16, df=1, p<0.02); male subjects had higher scores on factor 2 than did female subjects. It is important to note that factor 3, which was associated with age at onset and the presence of ADHD in the proband, was not associated with a diagnosis of OCD in the proband. Thus, this “compulsive” factor was not part of a diagnosis of obsessive-compulsive behavior.
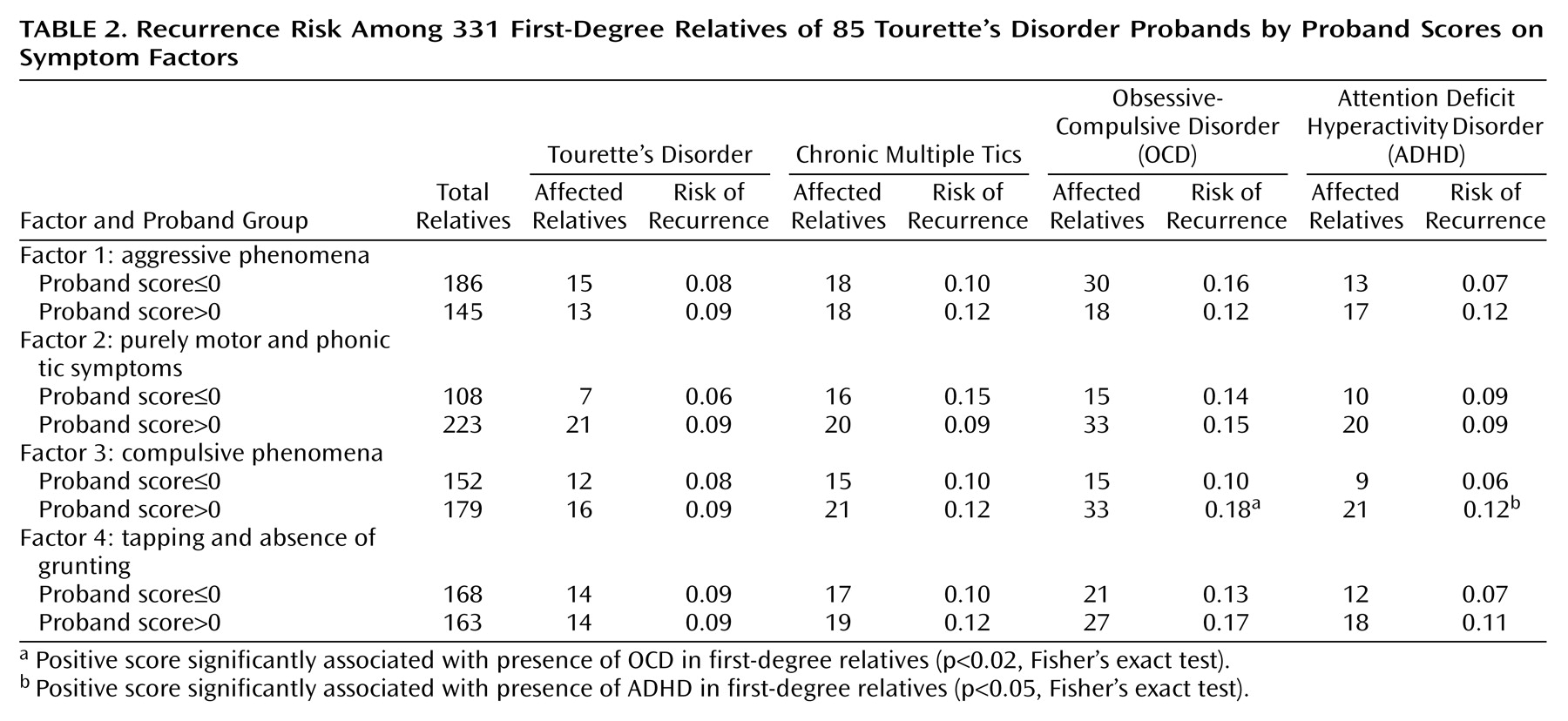
Next, analyses were completed to determine whether the factor scores were related to recurrence of illness in the relatives of Tourette’s disorder probands. The rates of illness are shown in
Table 2. High scores on the compulsive factor (factor 3) were significantly associated with both ADHD and OCD in the relatives. High scores on the aggressive factor (factor 1), while not reaching statistical significance (p<0.10, Fisher’s exact test), showed an association with a high recurrence of ADHD among the relatives. However, no factor was related to a differential risk of Tourette’s disorder among first-degree relatives.
The recurrence risks for Tourette’s disorder and chronic multiple tics across all families does not address the relationship between probands’ and relatives’ factor scores within families. To examine this, intraclass correlation coefficients were calculated for 36 independent proband-relative pairs. The correlation between the proband and relative factor scores was significant for three of the factors (factor 1: r=0.50 [F=1.99, df=35, 35, p<0.03]; factor 2: r=–0.07 [F=0.78, df=35, 35, p=0.59]; factor 3: r=0.46 [F=1.93, df=35, 35, p<0.04]; factor 4: r=0.52 [F=1.68, df=35, 35, p<0.02]).
Discussion
The principal-component factor analysis involving 85 probands with Tourette’s disorder revealed a significant underlying structure based solely upon the subjects’ observed and reported symptoms. We believe this is the first such report of significant factors found in a group of subjects with Tourette’s disorder.
The factor structure reported here appears to be a valid construct. Scores on a compulsion-like factor significantly predicted the age at onset of Tourette’s disorder. That same factor predicted comorbidity of ADHD in the probands and also predicted higher rates of ADHD and OCD among relatives. The ability of a model-free analysis to produce biologically relevant factors is an indication that the underlying symptom weightings represent an adequate description of a phenotypic subtype.
The delineation of an aggressive factor that included self-injurious behaviors and coprolalia is particularly interesting in light of a report by Robertson et al.
(3), who described a statistically significant relationship between the occurrence of copro-phenomena and self-injurious behavior. Here again it can be seen that an essentially model-free analysis resulted in a factor structure with clinically relevant symptom weightings.
Two factors (aggressive phenomena and compulsive phenomena) predicted comorbid ADHD in the probands. While the predictive power of factor 1 (aggressive phenomena) did not reach statistical significance, these two factors may represent distinct and specific subtypes of ADHD. The empirically derived factor 3 may be equivalent to one of the two DSM-IV components of ADHD, namely hyperactivity or impulsivity. The symptom loadings for repetitive speech and touching of others or objects for factor 3 fit a clinical picture of increased motor activity with decreased impulse control. This factor significantly predicted ADHD among relatives and appeared to be substantially heritable within families. It is surprising that proband scores on factor 3 also predicted the occurrence of OCD among their relatives. ADHD and OCD have been hypothesized to be related only as separate parts of a Tourette’s disorder spectrum; they are not generally seen as independently associated with each other. There may be identifiable shared components of the ADHD and OCD phenotypes, particularly as they relate to impulse control. It will be important to explore this possibility by examining factor scores in family samples of probands with ADHD and probands with OCD.
It was disappointing that neither factor 1 nor factor 3 appeared to be related to differential risk for Tourette’s disorder or chronic multiple tics among the relatives, since Santangelo et al.
(26) reported differences in familial risk for these disorders in Tourette’s disorder probands with “rage” tics or “compulsion” tics. However, those results were not based upon lifetime occurrences and may have been confounded with age at onset effects.
One explanation for the absence of any correlations with factor 2 (purely motor and phonic tic symptoms) is the lack of variability in the study group used to estimate the correlation. The symptoms included in factor 2 are necessary for a diagnosis of Tourette’s disorder or chronic tics while the other symptoms, although important when present, are not necessary for a diagnosis. It will be essential to get a better estimate of the normal variation for behaviors in factor 2 (perhaps through a general survey of unaffected individuals) and to determine if it can be divided into homogeneous components that show transmission.
The fourth factor, with a high positive loading for tapping and a large negative loading for grunting, remains something of a puzzle. The tapping symptom is distinct from other finger or hand motions and is a specific repetitive motor tic. We were unable to hypothesize a biologically relevant connection between such a movement and the absence of grunting. This finding may be an artifact due to the use of those symptoms as dichotomous variables in the factor analysis; both finger tapping and grunting remained as separate nonclustered variables after the hierarchical cluster analysis. However, the intraclass correlation of 0.52 for that factor argues against artifact.
Three of the four factors were correlated within families ascertained through Tourette’s disorder probands. The intraclass correlations within families for the aggressive, compulsive, and tapping/no grunting factors, ranging from 0.46 to 0.52, may indicate the existence of separate heritable components of the Tourette’s disorder phenotype. This could be useful in current searches for Tourette’s disorder susceptibility genes by treating each factor as an independent phenotype. Because environmental influences may contribute to intrafamily correlations, studies of these factors in Tourette’s disorder-affected twins would be an important approach for confirmation of these correlations.
Currently, most genome scans for linkage to psychiatric disorders employ a dichotomous affected/not affected phenotypic specification in the analysis phase. Clearly, there is a loss of information (and hence analytic power) when a disease state threshold is imposed upon a continuous phenotypic axis. Approaches to counter this loss of power have included varying the disease threshold for multiple serial univariate analyses or the use of a single composite variable. The existence of separate quantitative factors that may represent distinct heritable entities presents an opportunity for the use of multivariate quantitative trait linkage analysis
(27,
28). Such an analysis may extract more information than methods previously and currently used for Tourette’s disorder linkage analysis. A factor-based reanalysis of existing pedigrees and marker information may prove fruitful in the search for Tourette’s disorder-linked markers, along with the application of a factor-based approach to in-progress or future genome scans.
It should be noted that a potential methodological shortcoming of this study was the use of several residual dichotomous symptom variables that were not absorbed into any symptom cluster. Dichotomous variables are generally held to be incompatible with the factor analytic model
(29). There are specific instances where such usage can be justified, however. One such exception is the use of a purely heuristic set of criteria to search for any clustering patterns
(29). We believe that our heuristic approach coupled with the exploratory nature of the analysis justifies the inclusion of three dichotomous variables.
Perhaps the greatest strength of this study was the use of atheoretically derived symptom clusters, which resulted in a factor structure with clinically relevant symptom groupings among subjects in this study group. This lends strong support to the validity of the factor structure derived from this data set. Clearly more work is needed to refine our understanding of these factors. At a minimum, these findings need to be replicated in an independent sample that uses a more up-to-date symptom assessment (e.g., including premonitory urges). In order to facilitate comparisons with the present study, any new assessments must be able to reconstruct the specific tic symptoms described here.