About 5% of pregnant women meet the Research Diagnostic Criteria for major depression
(1), a suboptimal physiologic milieu for pregnancy. Antepartum depression may be a risk factor for development of preeclampsia
(2) and is the strongest predictor of postpartum depression
(3). Maternal depressive symptoms during pregnancy may lead to neonatal developmental changes and early childhood irritability
(4,
5). Further, an altered intrauterine climate may affect the newborn’s neurobehavioral function. Finally, infants of depressed mothers demonstrate lower basal and stimulated frontal EEG activity
(6).
Pilot studies have supported the potential value of interpersonal psychotherapy as a form of treatment for antepartum depression
(7) and explored the potential value of sleep deprivation or light therapy
(8). The safety of pharmacologic treatment of depression in pregnant women is controversial and just beginning to be the subject of controlled investigations
(9,
10). Treatment of depressed pregnant women with antidepressants requires skilled decision making based on limited data
(11). Exploration of alternative treatments for antepartum depression is therefore critically important.
Daily bright light exposure, alone or combined with antidepressant medications, has been shown in several small studies
(12,
13) to be effective in treating patients with nonseasonal major depression, and there are case reports of its potential value in treating postpartum depression
(14). Further studies of light therapy in combination with medication are required, however, to ascertain possible interactions and safety
(15). Pregnant women have been excluded from past studies of light therapy, however, to simplify study designs and avoid an uncharted dimension of risk assessment. To our knowledge, this study is the first to focus light treatment on this vulnerable population.
Method
Pregnant patients were recruited through the media and referrals from obstetricians. After complete description of the study to the patients, written informed consent was obtained. A diagnosis of major depressive disorder was established according to DSM-IV criteria
(16), and a Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorders Version (SIGH-SAD)
(17) score of at least 20 was required. Patients with the following characteristics were excluded from entry: current treatment with psychotropic medication; recent history of suicide attempt; panic or eating disorder unrelated to depression; other axis I diagnosis; current medical illness (including epilepsy, retinal pathology, cataract, or glaucoma); thyroid function test results inconsistent with normal pregnancy; past head injury with neurological or psychiatric sequelae; current use of β-adrenergic blockers, melatonin, or St. John’s wort; delayed-sleep-phase syndrome or hypersomnia with habitual sleep onset later than 1:00 a.m. and/or awakening later than 10:00 a.m.; and current use of alcohol or drugs (based on self-report).
The patients were followed for up to 2 weeks (observation period) before beginning treatment at a distance of 33 cm with an ultraviolet-screened diffuse white fluorescent light source (a Day-Light 10,000-lux box; Day-Light Technologies, Inc., Halifax, N.S., Canada) tilted downward toward the head of the seated patients. The patients were instructed to use the light therapy at home for 60 minutes daily, beginning within 10 minutes of awakening for at least 3 weeks. Compliance was documented by having the subjects call an answering machine daily to report their light use. A morning light time was chosen because of preponderant evidence that this interval elicits advances in the circadian rhythm phase that have been highly correlated with the effective treatment of winter seasonal depression
(18). The subjects were asked to maintain their habitual sleep schedules but to adjust them to an earlier time if necessary to accommodate a 1-hour morning light treatment session. At the end of the treatment trial, the patients stopped light therapy for a 1-week period. No patients were treated in the postpartum.
The patients returned to their clinics for weekly evaluations by psychiatrists and were given SIGH-SAD ratings by clinicians (psychiatrists, psychologists, and social workers) who were blind to treatment status and experienced with SIGH-SAD administration. The time of day of the evaluations was consistent among individual subjects but not across patients. Interrater reliability was addressed by consensus review of the rating criteria before, and every month during, the study. Changes in SIGH-SAD scores over time were evaluated by repeated measures analysis of variance with Greenhouse-Geisser correction
(19); post hoc t test comparisons of mean scores with Bonferroni correction were performed for each week. Adverse effects were monitored by using the Systematic Assessment for Treatment Emergent Effects
(20), and the subjects were also asked to report any distinct occurrence in the week preceding each evaluation session. Symptoms of mania or hypomania were explored by the evaluating psychiatrists weekly.
We obtained global seasonality scores
(21) to assess the degree of historical seasonal variation of mood and energy. We performed Pearson’s correlations between degree of seasonality, week of gestation, proportion of the baseline depression score made up of atypical symptoms of depression (e.g., hypersomnia and carbohydrate cravings), and the percentage of improvement on the SIGH-SAD at 3 weeks. After delivery we sent each patient a questionnaire to assess birth outcome and the infants’ health status.
Results
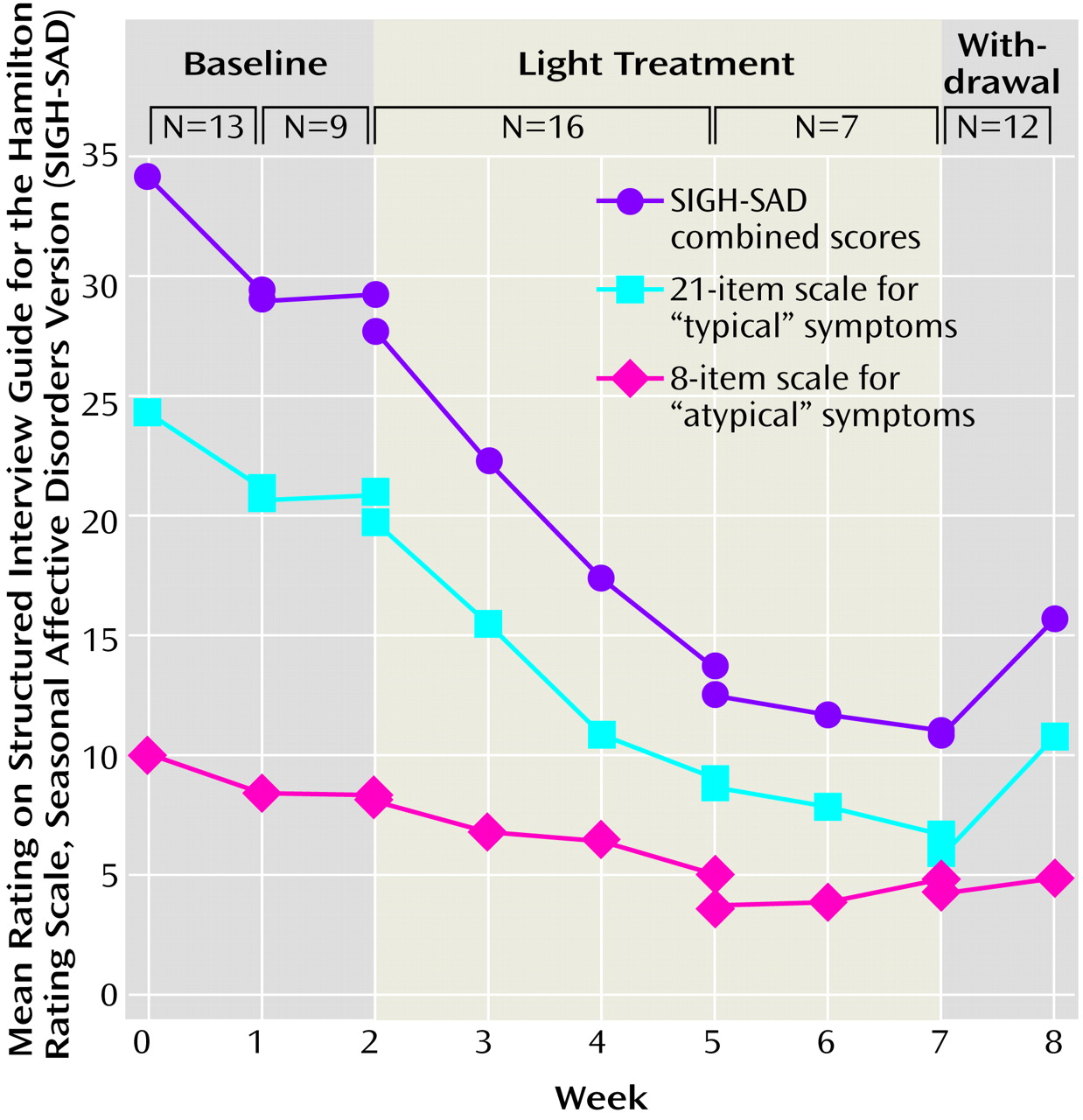
Eighteen outpatients entered the study. Five patients were immediately assigned to treatment. The remaining 13 patients were deemed eligible and were willing to participate in a baseline week of SIGH-SAD assessment and no treatment before beginning treatment; nine of these patients were willing to participate in the 2 baseline weeks of assessment. One week of baseline observation had minimal effect (improvement: mean=13%, SD=22%) on SIGH-SAD scores, while a second week of observation produced no further improvement in the group of nine patients (
Figure 1). Two subjects were excluded from analysis. One of them (with a previous stillbirth) experienced another stillbirth 1 week after commencing light therapy and withdrew from the protocol; another patient was noncompliant. The demographic characteristics of the 16 remaining study subjects were as follows: age, mean=34 years (SD=4); all Caucasian; gravity, mean=3 (SD=1); parity, mean=1 (SD=1). Fourteen patients had a past history of major depressive disorder before pregnancy; two of these had winter seasonal depression; and none had a history of dysthymia. Their mean global seasonality score
(22) was 8 (SD=6) (two scores were missing), which is indicative only of “slight” seasonal mood variation. Gestation at the start of treatment was 23 weeks (SD=7).
Sixteen patients completed the 3-week protocol, and seven patients completed 2 additional weeks of the study. Twelve patients were observed for a 1-week “withdrawal” period after light treatment. Although the patients were eligible for treatment throughout the year, the timing of patient entry was such that 75% (12 of 16) entered treatment between November 6th and February 6th. Two patients experienced nausea as a side effect, which was largely ameliorated in one case with a reduction of daily light exposure to 45 minutes. No other significant side effects were reported.
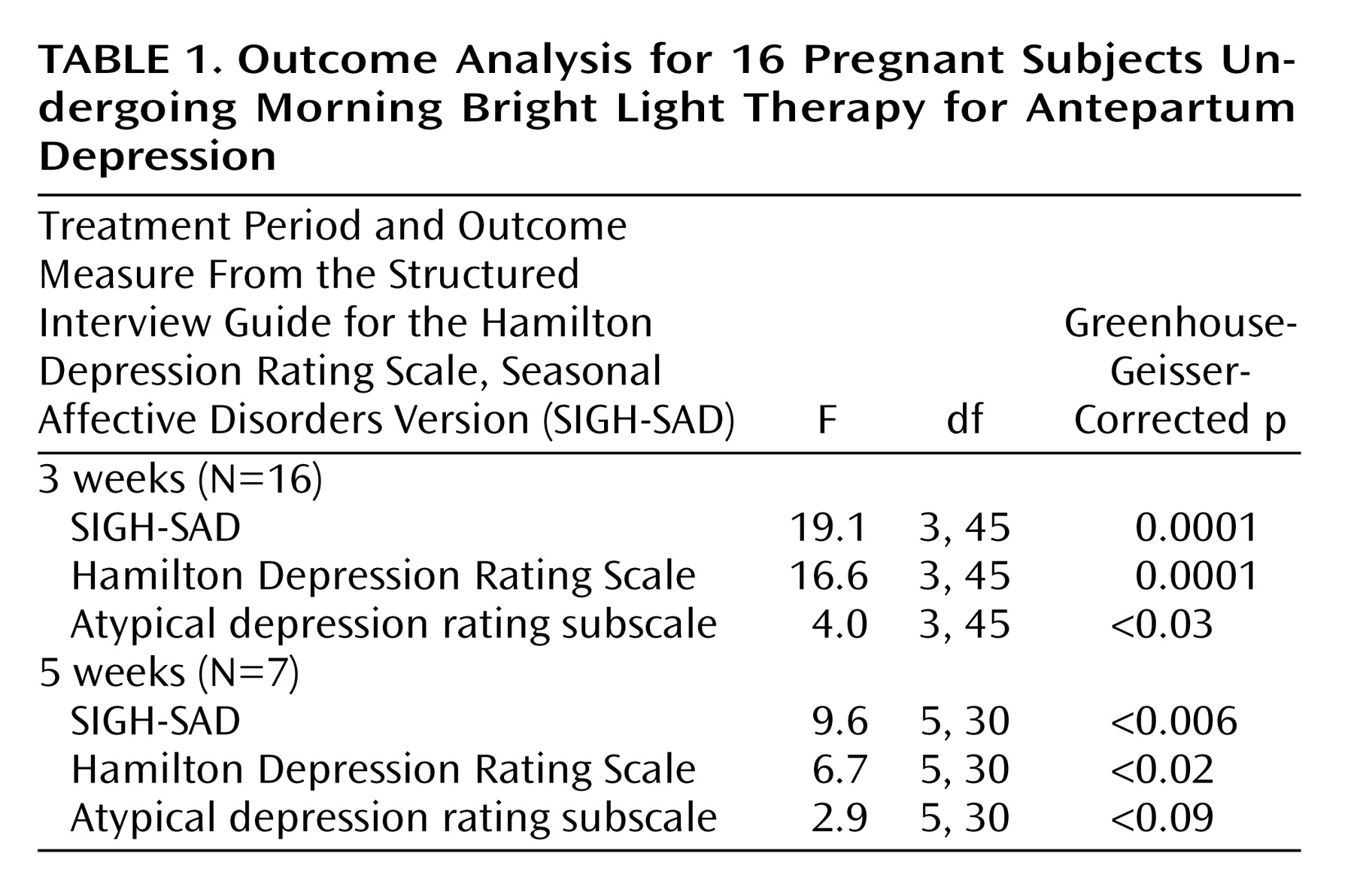
In the 16 patients treated for 3 weeks, SIGH-SAD depression ratings had improved moderately (by 49%) after 3 weeks (improvement between weeks 0 and 2: t=4.49, df=15, p<0.001; improvement between weeks 0 and 3: t=6.27, df=15, p<0.001; and improvement between weeks 1 and 3: t=4.76, df=15, p<0.005; all with Bonferroni correction) (
Table 1). After observing these responses in the first nine patients who completed 3 weeks of treatment, we lengthened the study to 5 weeks. For the seven subjects followed during 5 weeks of light treatment, mean scores on the SIGH-SAD for these subjects improved by 59% from baseline (improvement between weeks 0 and weeks 2, 3, 4, and 5: t=4.00–4.40, df=6, p<0.05, with Bonferroni correction). After 3 weeks, eight of the 16 subjects had a decrease in their SIGH-SAD scores by at least 50%. After 5 weeks of treatment, four of seven subjects achieved a decrease in their SIGH-SAD scores by at least 50%, as well as a final SIGH-SAD score less than 8, which we defined as “complete remission.” Of the seven who completed 5 weeks of treatment, three had complete remissions after 3 weeks, and four achieved complete remission by the fifth week. During the 1 week of withdrawal of light therapy, SIGH-SAD scores increased by 5 (SD=10) in the 12 patients we observed. Five of these 12 patients had an increase in their SIGH-SAD scores by at least 5 points.
There was no significant correlation between improvement in SIGH-SAD scores after 3 weeks and week of gestation (r=0.02, p>0.93, N=16) or between improvement on SIGH-SAD scores and baseline score on the atypical depression portion of the SIGH-SAD (atypical depression score divided by total SIGH-SAD score: r=0.14, p=0.60, N=16). Furthermore, there was no significant correlation between seasonality scores and improvement on the SIGH-SAD after 3 weeks of treatment (r=0.10, p=0.73, N=14; data for two patients were missing).
Fourteen of the 16 patients returned the follow-up questionnaire. One mother had one male and one female twin; the other mothers produced seven boys and six girls. Continued light therapy provided beneficial effect in the treatment responders who used the treatment after the trial and throughout the remainder of the pregnancy. After completion of the study, depression returned during pregnancy for four of the 14 patients, within 3 months of delivery for another four, and later for two more patients. Nine had normal vaginal deliveries; five had Cesarean sections. Mean birth weight was 110 oz (SD=26). Only one of the mothers did not gain weight at a normal pace for her date of pregnancy; her son was born at 30 weeks. Another infant was born prematurely at 32 weeks. In the six children in which it was known, the mean Apgar score at birth was 8 (SD=2). All 15 children were reported to have experienced “normal” weight gain, growth, and attainment of milestones.