The prevalence of obesity in the general population of the United States has reached 19.8% and continues to increase across all sociodemographic groups
(1). This “epidemic” is of great concern considering related morbidity and mortality rates. Major medical problems linked with obesity include hypertension and cardiovascular disease, dyslipidemia, diabetes mellitus, gallbladder disease, osteoarthritis, gallstones, musculoskeletal problems, and selected cancers
(2–
7). Among U.S. adults, the estimated number of annual deaths directly attributable to obesity is approximately 280,000. Obesity-related morbidity accounts for an estimated 6.8% of U.S. health care costs, and the economic costs of illnesses associated with obesity amount to approximately $68 billion per year. In addition, U.S. adults annually spend $30 billion on weight-reduction programs and foods
(8).
Individuals suffering from bipolar disorder may be particularly at risk for obesity
(9). We recently reported on weight changes during acute and maintenance treatment observed in 50 patients with bipolar I disorder who were treated for at least 12 months after remission of their index affective episode without experiencing a recurrence
(9). We found that, for this group, 1) the prevalence of obesity greatly exceeded that found in the general population (32% versus 19.8%, respectively); 2) despite patients’ receiving pharmacotherapy in both phases of treatment, the largest increases in weight occurred during acute treatment as opposed to the maintenance treatment phase; 3) the amount of weight gain during the acute treatment phase was positively correlated with baseline scores on the Hamilton Rating Scale for Depression and negatively correlated with baseline scores on the Bech-Rafaelsen Mania Scale; 4) the number of previous depressive episodes significantly contributed to the likelihood of being overweight or obese at study entry; and 5) there was a negative relationship between body mass index and the tendency to gain weight, during both acute and maintenance treatment.
This present report examines the relationships between obesity and several indicators of severity of illness, including the likelihood of a recurrence, among the entire group of 175 patients with bipolar I disorder who were treated in the Maintenance Therapies for Bipolar Disorder study
(10). We hypothesized that baseline demographic and clinical characteristics and treatment outcomes for patients deemed obese would differ from those of nonobese patients. Our hypotheses were based on our clinical observations during the trial, clinical experience with patients with bipolar I disorder over the years, and several published reports concerning the relationship between obesity and poorer quality of life
(11,
12), social life
(13,
14), psychological well-being
(12,
15,
16), self-esteem, general physical well-being, and overall functioning
(1,
11,
13,
14,
17).
Method
The Maintenance Therapies for Bipolar Disorder protocol is a randomized controlled trial comparing interpersonal and social rhythm therapy to an intensive clinical management approach in patients affected by bipolar I disorder
(10). Patients in both treatment groups (interpersonal and social rhythm therapy and intensive clinical management) receive pharmacotherapy
(9). To enter the study, patients must have a lifetime diagnosis of bipolar I disorder (confirmed by the Structured Clinical Interview for DSM-IV or, in the early part of the trial, by the Schedule for Affective Disorders and Schizophrenia) and be in the midst of at least their third affective episode, with the most recent episode being within 5 years of the index episode. Patients must be between 18 and 65 years old.
In the Maintenance Therapies for Bipolar Disorder study, we attempted to stabilize the maximum number of patients possible with lithium monotherapy. Patients who could not tolerate lithium received valproic acid or carbamazepine. Patients with major depression whose illness did not stabilize with lithium alone received adjunctive tranylcypromine or, if they were unwilling to take a monoamine oxidase inhibitor (MAOI), they received paroxetine or another antidepressant. Patients with manic or psychotic symptoms whose condition did not stabilize with lithium alone received an adjunctive neuroleptic.
An independent evaluator assessed the patients’ clinical states at each visit with the Bech-Rafaelsen Mania Scale
(18) and the 25-item Hamilton Rating Scale for Depression
(19). The 25-item Hamilton depression scale is an adaptation of the original 17-item Hamilton depression scale and includes the original items plus eight additional items that evaluate reverse neurovegetative symptoms
(20).
The Maintenance Therapies for Bipolar Disorder protocol is divided into an acute treatment phase and a maintenance treatment phase. Patients are seen weekly in the acute phase, biweekly for the first 3 months of the maintenance phase, and then monthly for the remaining 21 months of the maintenance phase. Patients are considered to have completed the acute phase when they achieve a 4-week period of remission, as defined by a mean score of ≤7 on the 17-item Hamilton depression scale and a mean score of ≤7 on the Bech-Rafaelsen Mania Scale. Unless a patient experiences a recurrence, no changes in the medication regimen are made after the acute phase.
For this report, we adopted the body mass index weight classification criteria published by the World Health Organization and accepted and amplified by the International Obesity Task Force
(21). Body mass index is calculated as weight (kilograms) divided by height (meters squared). Individuals are considered underweight if their body mass index is less than 18.50, of normal weight if their body mass index is 18.50–24.90, overweight if their body mass index is 25.00–29.90, and obese if their body mass index is equal to or greater than 30.00. For this report, individuals were classified as nonobese if their body mass index was less than 30.00 and obese if their body mass index equaled or exceeded 30.00.
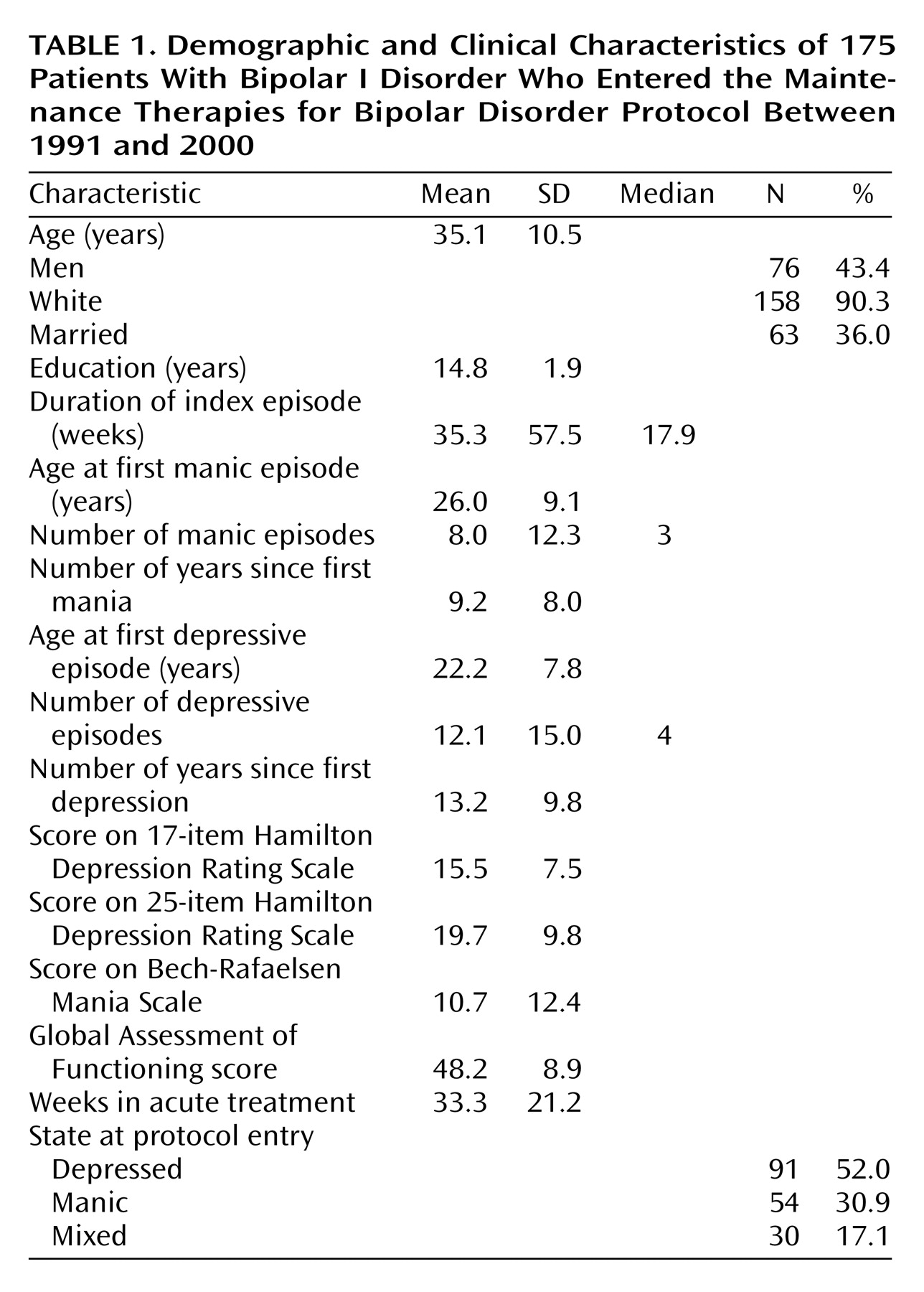
The study group for this report included 175 patients with bipolar I disorder who enrolled in the Maintenance Therapies for Bipolar Disorder study from 1991 to 2000. Their demographic and clinical characteristics are presented in
Table 1. Data on lifetime use of neuroleptics were obtained from National Institute of Mental Health life-chart protocols
(22). The University of Pittsburgh’s biomedical institutional review board approved all recruitment, assessment, and treatment procedures. All subjects who met inclusion and exclusion criteria provided written informed consent after receiving a complete description of the study and having an opportunity to ask questions.
Baseline demographic and clinical characteristics of the patients were compared across the two weight groups (obese versus nonobese) by using chi-square analysis for contingency tables on categorical data and group t tests on continuous data. Time to recurrence was compared across the two weight groups by using Kaplan-Meier survival analysis. To control for baseline differences among the groups, a Cox survival regression model was used, with body mass index used as a continuous variable and scores on the 25-item Hamilton depression scale and number of previous depressive episodes as covariates.
Results
A total of 35.4% (N=62) of the 175 patients met criteria for obesity. For obese patients, median height was 66 inches, and median weight was 212 lb. For nonobese patients, median height was 67 inches, and median weight was 159 lb. Among the 62 obese patients, 20 individuals (32.3%) received a typical neuroleptic, and six individuals (9.7%) received an atypical neuroleptic at some point in their lifetimes before they entered the Maintenance Therapies for Bipolar Disorder protocol. The median numbers of days taking typical and atypical neuroleptics were 294 and 312, respectively. Among the 113 nonobese patients, 33 individuals (29.2%) received a typical neuroleptic, and three patients (2.7%) received an atypical neuroleptic during their lifetimes. The median numbers of days taking typical and atypical neuroleptics were 343 and 195, respectively. No statistically significant difference was found between the groups for percentage of patients taking typical neuroleptics, percentage of patients taking atypical neuroleptics, or number of days taking neuroleptics.
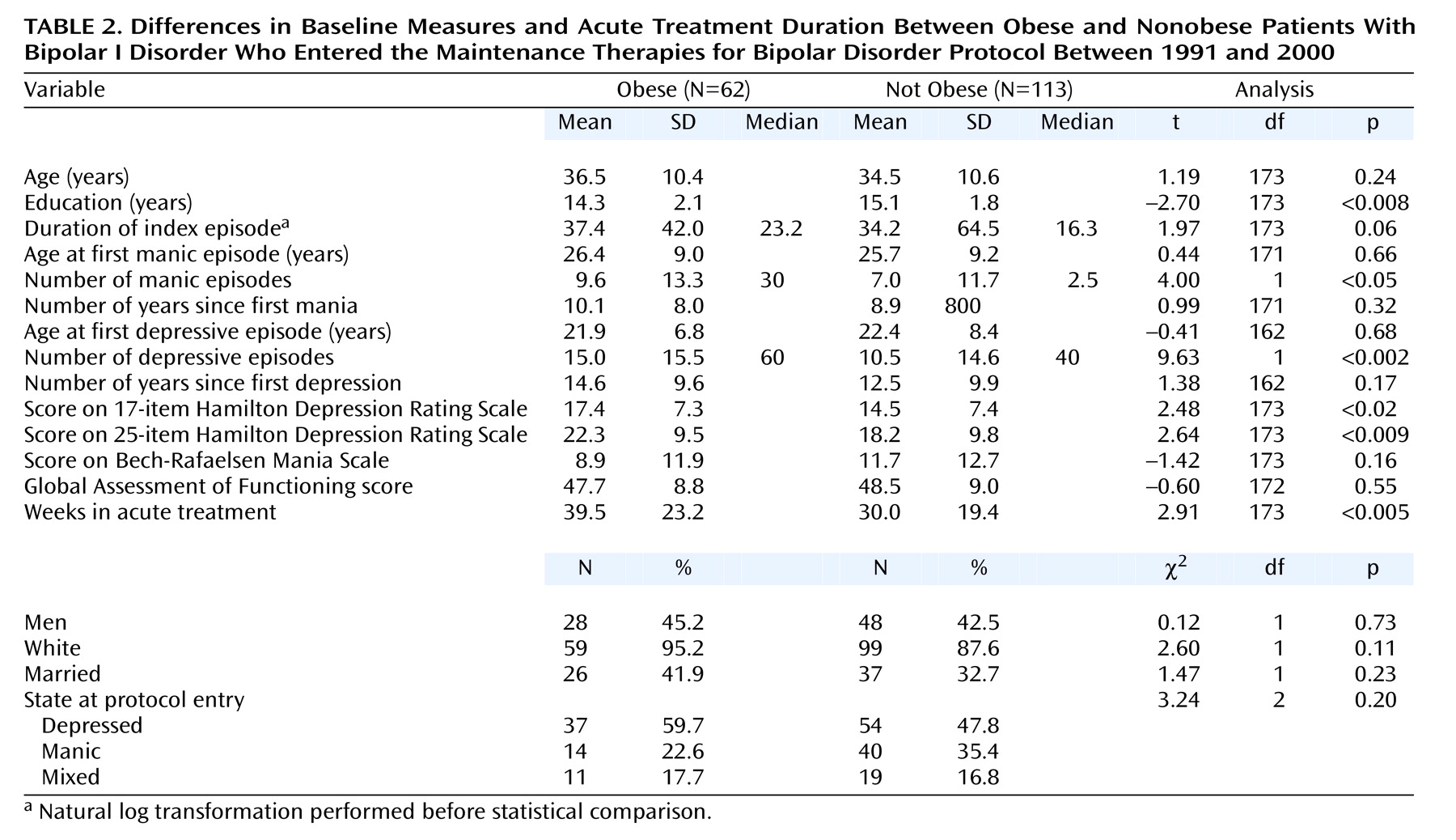
The obese group had significantly fewer years of education (t=–2.70, df=173, p<0.008), more previous depressive episodes (t=9.63, df=1, p<0.002), more previous manic episodes (t=4.00, df=1, p<0.05), and higher baseline 17-item (t=2.48, df=173, p<0.02) and 25-item (t=2.64, df=173, p<0.009) Hamilton depression scale scores, and they required more time in acute treatment (t=2.91, df=173, p<0.005) to achieve stable remission (
Table 2). A total of 74.2% (N=46) of the obese subjects completed the acute phase of treatment compared to 70.0% (N=79) of the nonobese subjects.
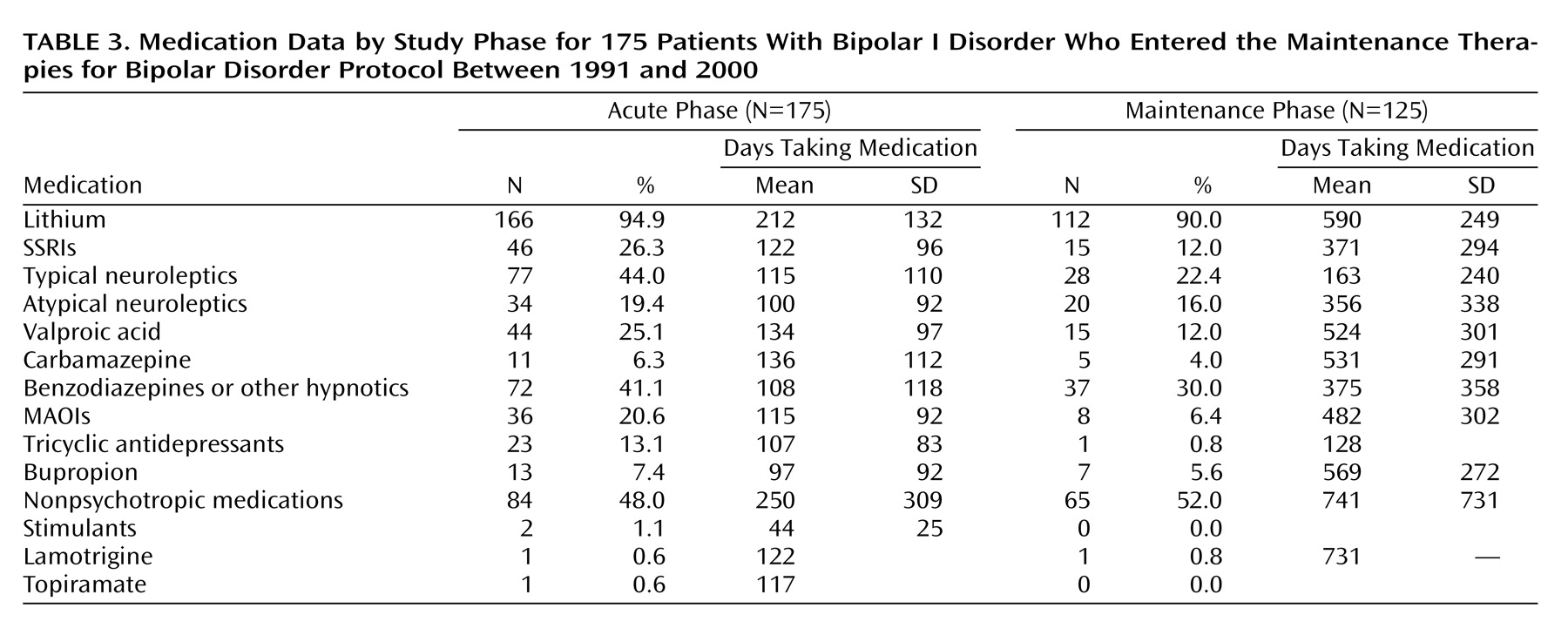
Table 3 shows the medications that were used during the trial. Most of the patients (94.9% [N=166] during acute treatment and 90.0% [N=112] during maintenance treatment) were treated with lithium as a mood stabilizer. No significant difference was found between the obese and the nonobese patients for lithium level during acute treatment (mean=0.81 meq/liter, SD=0.13; mean=0.80 meq/liter, SD=0.16, respectively) or maintenance treatment (mean=0.83 meq/liter, SD=0.12; mean=0.80 meq/liter, SD=0.17, respectively). A limited number of patients received valproic acid (25.1% [N=44] during acute treatment and 12.0% [N=15] during maintenance treatment) or carbamazepine (6.3% [N=11] during acute treatment and 4.0% [N=5] during maintenance treatment) alone or in combination with lithium. A significant difference was found between the obese and the nonobese patients’ valproic acid levels during acute (t=3.98, df=40, p<0.001) and maintenance (t=2.27, df=12, p<0.05) treatment. During acute treatment, the mean levels of valproic acid were 52 mg/ml (SD=18) for the obese and 72 mg/ml (SD=15) for the nonobese patients. During maintenance treatment, the mean levels of valproic acid were 61 mg/ml (SD=10) for the obese and 75 mg/ml (SD=12) for the nonobese patients.
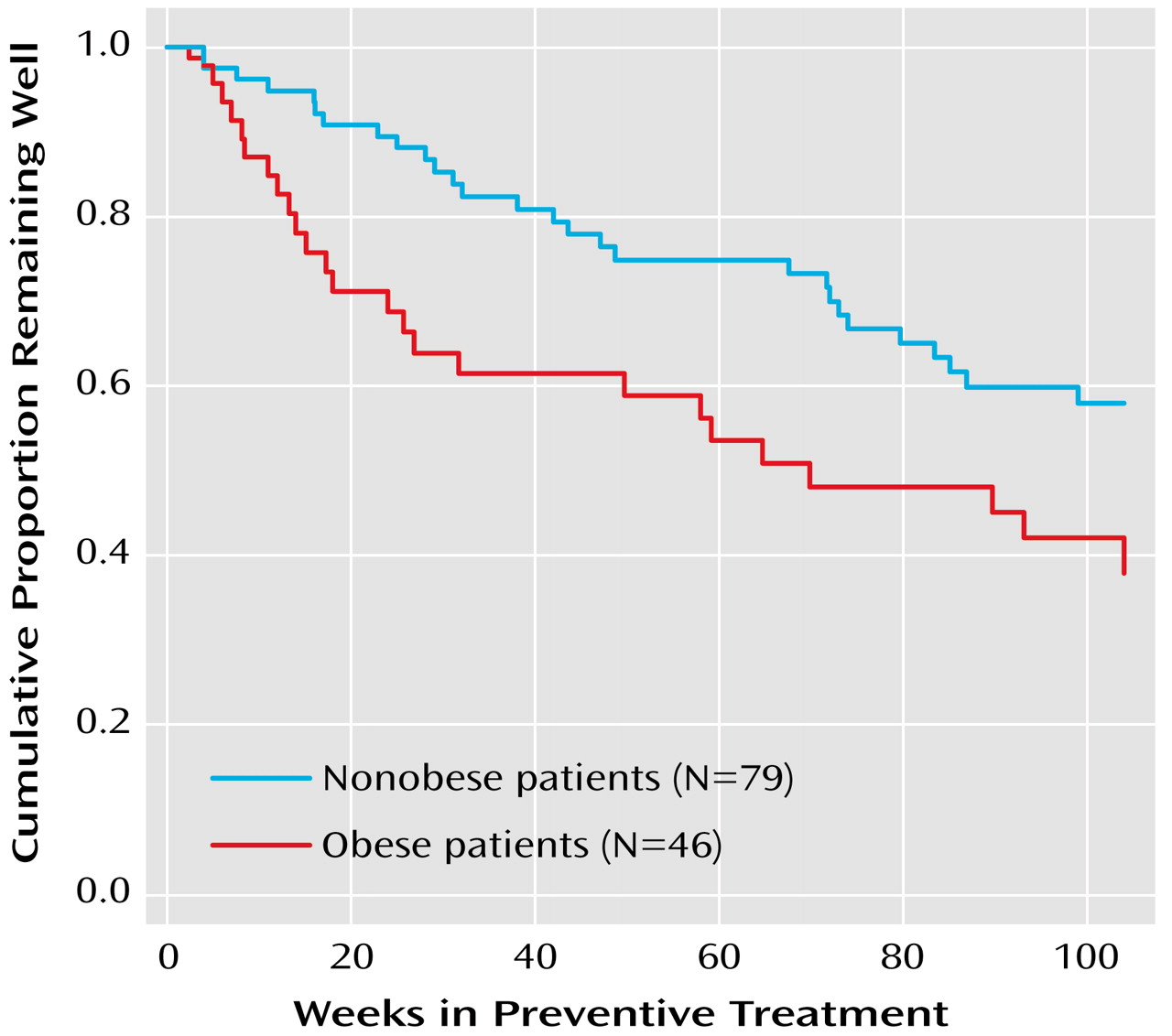
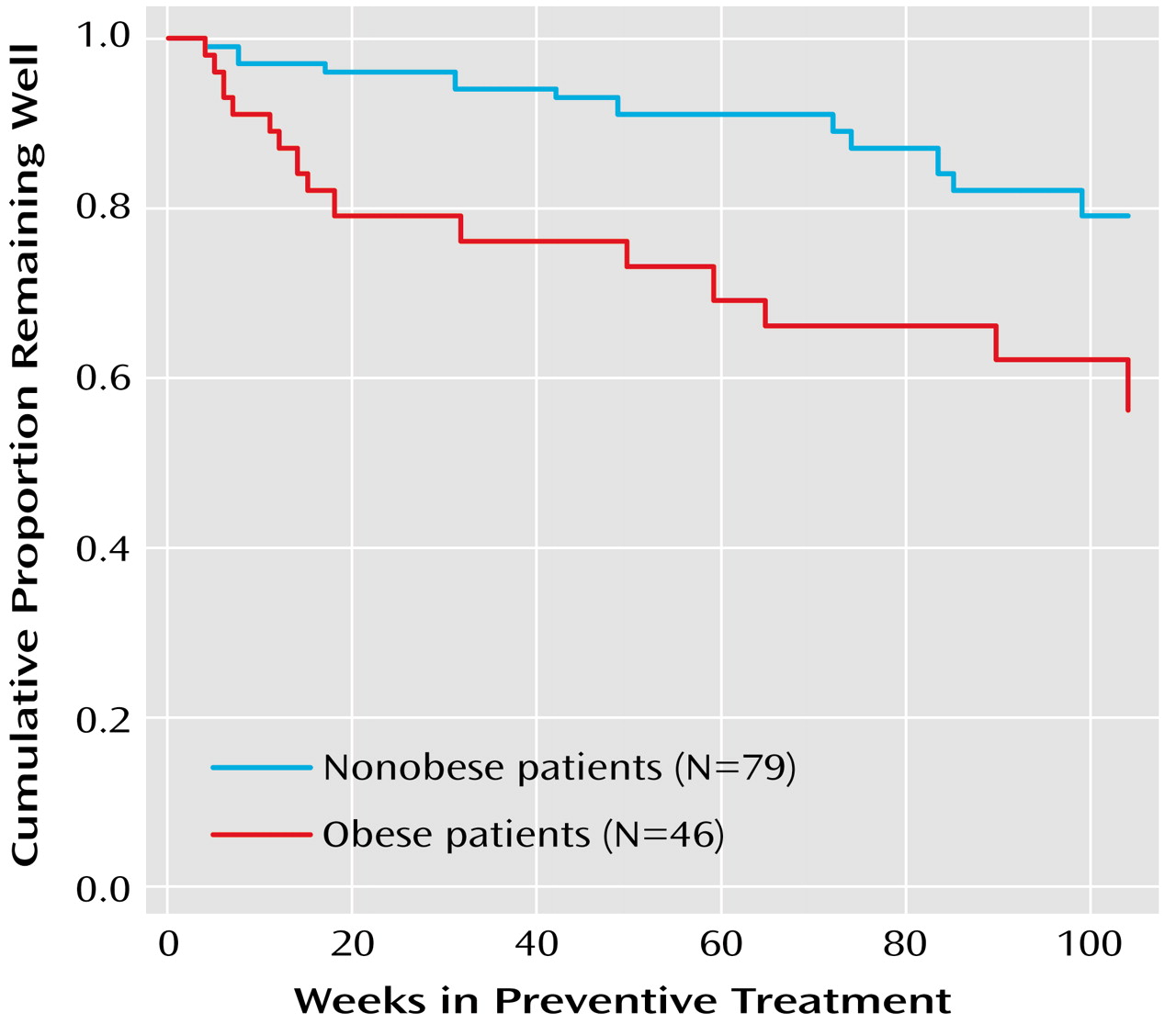
Among the 125 patients who completed the acute treatment phase and entered the preventative maintenance phase, the percentage experiencing a recurrence was higher in the obese group (54.3%, N=25) than in the nonobese group (35.4%, N=28) (χ
2=4.25, df=1, p<0.04). A Kaplan-Meier survival analysis confirmed that the time to recurrence for patients who were obese at baseline was significantly shorter than that of patients who were not obese at baseline (log rank χ
2=5.54, df=1, p<0.02) (
Figure 1). With control for the number of depressive episodes, number of manic episodes, and baseline Hamilton depression scale scores, body mass index remained a statistically significant predictor of time to recurrence (hazard ratio=1.06, χ
2=6.11, df=1, p<0.02). The percentage of patients experiencing depressive recurrences was significantly greater in the obese group than in the nonobese group (32.6% [N=15] versus 13.9% [N=11]) (χ
2=6.16, df=1, p<0.02). Not surprisingly, a Kaplan-Meier survival analysis confirmed that time to depressive recurrence was significantly shorter in the obese group (log rank χ
2=7.33, df=1, p<0.007) (
Figure 2). With control for the number of depressive episodes and baseline Hamilton depression scale scores, body mass index remained a statistically significant predictor of time to depressive recurrence (χ
2=5.73, df=1, p<0.02). The difference in number of manic or mixed recurrence episodes between the obese and the nonobese groups was not statistically significant.
Discussion
This study suggests that obesity is correlated with important clinical features of patients with bipolar I disorder. We found that obese patients 1) experienced a greater number of lifetime depressive and manic episodes, 2) presented with a more severe and difficult-to-treat index affective episode, and 3) were more likely to develop an affective recurrence and, in particular, a depressive recurrence.
Obesity has psychosocial consequences, including discrimination and stigmatization in multiple areas of daily life, such as health care, education, and employment
(11,
13,
14). Obesity may contribute to the severity of bipolar disorder through several factors, including its negative impact on general physical well-being and functioning
(1,
11,
13,
14,
17), quality of life
(11,
12), self-esteem, and psychological well-being
(12,
15,
16). Obesity may also contribute to sleep apnea, which may disrupt sleep and other circadian rhythms, thus causing or contributing to mood destabilization
(23,
24).
Obesity can alter the distribution and elimination of drugs and therefore may contribute to the severity of bipolar disorder if the loading and/or maintenance doses of the drugs are not appropriately modified
(25). However, our finding of differences in valproic acid blood levels between obese and nonobese patients should be interpreted with caution, given the limited number of patients who were treated with valproic acid and that a number of those patients (36 of 44 during acute treatment and six of 15 during maintenance treatment) were receiving a combination of lithium and valproic acid. Moreover, although the knowledge of the influence of obesity on drug pharmacokinetics is surprisingly limited and conflicting
(25), some reports have suggested that obesity does not affect the serum concentrations of a hydrophilic drug such as valproic acid
(26).
Conversely, bipolar disorder may increase the risk of obesity due to medication exposure and disease-specific symptoms that commonly occur during long-lasting depressive episodes, such as increases in appetite and reduced energy expenditure.
These observations, along with the present finding that obese patients are at a higher risk for rapid recurrence, may have important clinical implications. For instance, they call into question—especially for obese patients—the practice of discontinuing treatment with antidepressant medications after remission of an acute depressive episode.
The prevalence of obesity in this study group was much higher (35.4%) than the prevalence in the general U.S. (19.8%) or local (20.7% in the Commonwealth of Pennsylvania) population
(1), confirming our previous finding that the prevalence of obesity among patients with bipolar I disorder is alarmingly high
(9). Obesity in patients with bipolar I disorder thus constitutes a major public health problem and suggests that the development and testing of specific interventions that target the obesity epidemic in this particular population are urgently needed. Bipolar disorder and obesity both have a tremendous impact on the physical and mental well-being of affected individuals. Therefore, both illnesses should be treated with a coordinated intensive and multifaceted treatment.
Losing weight is a challenge, particularly for patients with a mental illness
(23). Early intervention is the key to preventing obesity in this population
(27). Ideally, diet and exercise counseling should be provided to all bipolar disorder patients—before weight gain—and definitely provided once weight gain has occurred. Exercise, diet, and individual or group behavioral therapy are the principal nonpharmacological means for inducing and maintaining weight loss. However, these may be particularly difficult to implement in individuals suffering from mood disorders.
In cases in which weight gain is clearly related to a specific medication, one option is switching to another medication or, when possible, decreasing the drug dose. However, these decisions are difficult, especially when the medication potentially liable for the weight gain has been effective at ameliorating symptoms and improving the overall quality of life for the patient.
According to guidelines for the general population, pharmacotherapy for obesity should be reserved for patients with a body mass index of >30 kg/m
2 or for patients with a body mass index of >27 kg/m
2 and risk factors for cardiovascular disease, stroke, or diabetes
(27). Surgical treatment is an option for patients with a body mass index that exceeds 40 kg/m
2 and who have not responded to more conservative measures to reduce weight
(27). Although studies have found that pharmacotherapy and surgical treatments for obesity are efficacious and relatively safe, we are not aware of any study in which these treatments have specifically been tested on patients with bipolar disorder.
Obesity is correlated with several indicators of a worsening prognosis and outcome in bipolar I disorder. Preventing and treating obesity in bipolar disorder patients could decrease the morbidity and mortality related to physical illness, enhance psychological well-being, and possibly improve the course of bipolar illness. We strongly support the development and testing of weight-control interventions specifically designed for patients with bipolar disorder and the implementation of these interventions in the routine care of patients with this illness.