In previous studies, the power to detect differences in hippocampal structure between depressed patients and healthy subjects may have been limited by exclusive reliance on the analysis of hippocampal volume. It is possible that depressed patients have structural abnormalities that are too subtle or specific to be reflected in measures of overall volume. Potentially, analysis of the shape of this brain structure could represent a more powerful approach to identifying such abnormalities.
Method
Depressed patients and comparison subjects were recruited by advertisement. Each subject had a psychiatric interview with a psychiatrist, and the Structured Clinical Interview for DSM-IV
(25) was administered. A medical history was taken, and a physical examination was conducted by a physician. The 21-item Hamilton Depression Rating Scale
(26) and Clinical Global Impression (CGI) scale
(27) were also administered to the depressed patients. All subjects were required to have no history of neurological illness, mental retardation, head injury, diabetes, or cardiovascular disorders, including hypertension. The depressed patients were also required to have a DSM-IV diagnosis of unipolar major depressive disorder in a current episode of at least moderate severity, a score of at least 21 on the Hamilton depression scale, a score of at least 7 on the eight items of the Core Endogenomorphic Scale of Thase et al.
(28) that are included in the Hamilton depression scale, no electroconvulsive therapy in the previous 6 months, and no drug or alcohol abuse in the previous 3 months. In addition to meeting the requirements for all subjects, the comparison subjects were required to have no lifetime history of axis I or axis II disorders. The depressed and comparison groups were matched for gender distribution and age. After complete description of the study to the subjects, written informed consent was obtained.
MRI scanning was conducted at the Mallinckrodt Institute of Radiology by using a Magnetom SP-4000 1.5-T imaging system (Siemens Medical Systems, Iselin, N.J.) and a turbo fast low-angle shot (FLASH) sequence with TR=20 msec, TE=5.4 msec, flip angle=30°, acquisitions=1, matrix=256×256, scanning time=13.5 minutes. This sequence allowed acquisition of three-dimensional data sets with 1-mm3 isotropic voxels. Further processing of the raw MRI data was conducted with Analyze software. To maximize contrast between white matter and CSF, signed 16-bit data sets were transformed to unsigned 8-bit data sets by using linear interpolation of all voxel intensities with the corpus callosum and lateral ventricle set as limiting values.
High-dimensional brain mapping was conducted according to the principles described in the introduction. The neuroanatomical template was the MRI scan of a healthy subject not included in the study group, and it was the same template used in previous studies by our group
(20,
21). In this template, the left and right hippocampi were outlined manually by expert consensus
(20,
21) according to neuroanatomical guidelines used previously
(20,
21,
24). For each target scan in the two study groups, landmarks were placed at the external boundaries of the brain, at the points where the anterior and posterior commissures intersect the midsagittal plane, and at selected points on the hippocampal surface proceeding along the anterior-posterior axis of the hippocampus
(24). The transformation of the template to match each target occurred as a two-step procedure. First, an automated procedure was used to coarsely align the template to the target on the basis of the landmarks; the mathematical basis of this initial transformation has been described elsewhere
(29,
30). In the second step, each voxel in the template was transformed to match an analogous voxel in the target, by using the model in which voxels within a scan have physical properties of a fluid
(15,
17–19); this second step was also an automated procedure. It has been shown previously
(23,
24) that delineation of the neuroanatomical boundaries of the hippocampus by high-dimensional brain mapping does not change with variations in landmark placement by raters.
A triangulated graph of points was superimposed onto the hippocampal surface in the template by using the Marching Cubes algorithm, as described previously
(30,
31); this procedure generated a graphical surface with more than 16,000 points. This graphical surface was carried along in the transformation of the template onto each target scan, and the displacement of each point on the hippocampal surface from template to target was represented as a vector. The volume of the hippocampus was estimated by computing the volume enclosed by the transformed hippocampal surface. The complete set of transformation vectors for all subjects together was subjected to principal components analysis, and a priori the first 10 components were taken to represent hippocampal shape; the choice of this number of components was based on the numbers of components that have been found useful for characterizing hippocampal shape in studies of other psychiatric disorders
(20–
22). Total brain volumes were computed by elastic-based transformations according to a procedure that has been described previously
(19).
Group comparisons on hippocampal shape and volume indexes were conducted after we verified that the 10 components and the volume indexes had similar variances across the two groups and that, within each group, these variables had distributions that did not depart markedly from normality. The hippocampal shapes of the depressed patients and comparison subjects were compared by means of a multivariate analysis of variance (MANOVA) conducted on the 10 components together. The linear discriminant function associated with the MANOVA (i.e., the linear combination of the 10 components that best discriminated the two groups) was computed to represent the ability of the shape indexes to classify individual subjects. This discriminant function was based on a model defined a priori (i.e., the set of 10 components), and this analysis is distinct from stepwise discriminant procedures that choose variables for the model only after assessing their ability to predict group membership. Univariate analysis of variance (ANOVA) was used to compare depressed patients and comparison subjects on left and right hippocampal volume and total brain volume.
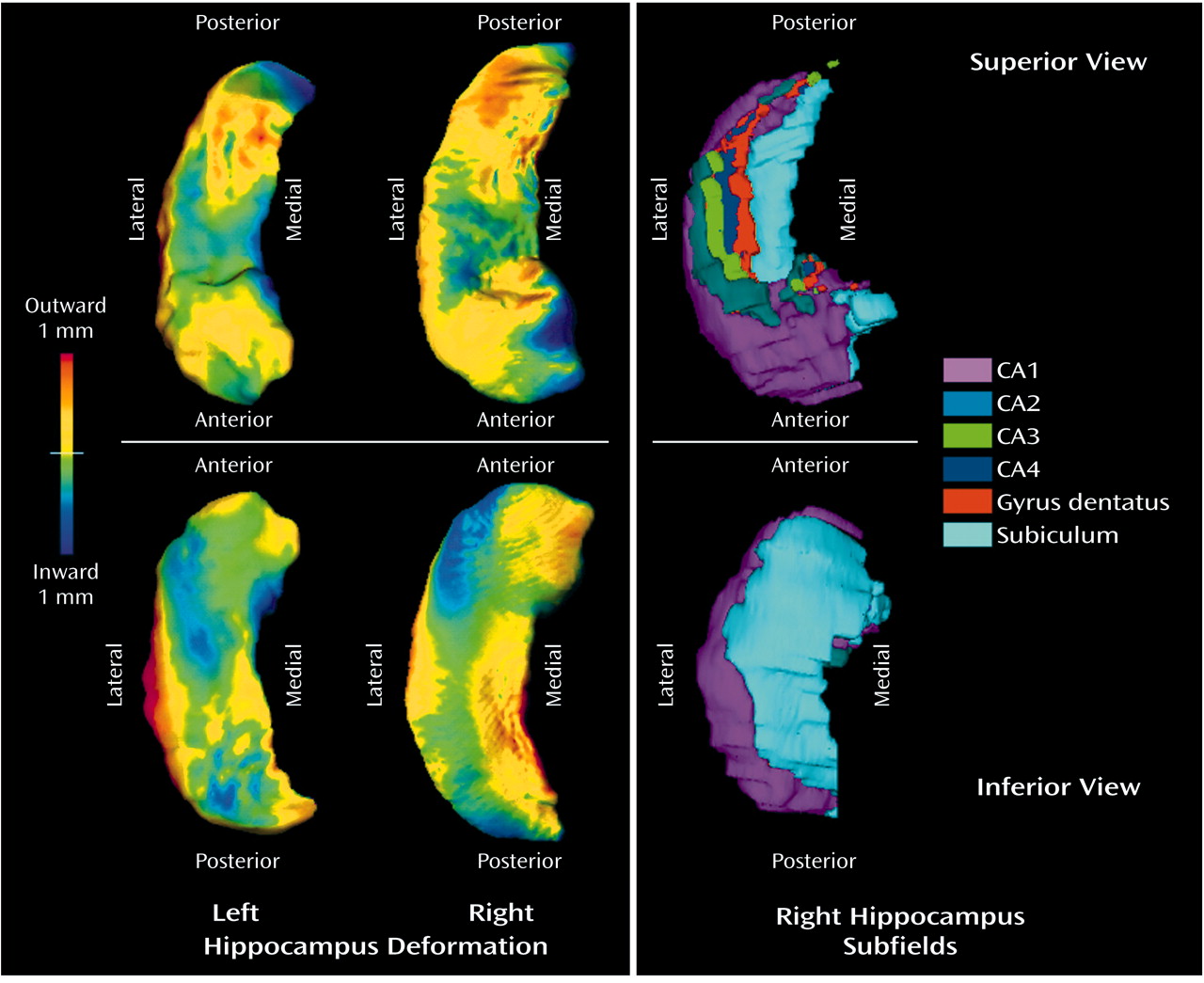
To display graphically the difference in hippocampal shape between depressed patients and comparison subjects, maps of the hippocampal surface were constructed on the basis of the 10 components taken to represent hippocampal shape. The averaged surface for the comparison group was displayed, and the average displacement at each point in the depressed group relative to the comparison group was color coded. Similar methods of displaying hippocampal surface deformities associated with disease conditions have been used previously in studies of schizophrenia
(20,
21) and Alzheimer’s disease
(22). To provide a comparative illustration of the normal hippocampal surface in terms of the underlying hippocampal subfields, an MRI scan from a healthy subject not included in the study groups was selected, and the subfields were outlined manually by an expert neuroanatomist (M.H.G.) on the basis of subfield anatomy represented in standard atlases
(32,
33). A map of the hippocampal surface coded by the underlying subfield in this healthy subject was then generated. These graphical presentations were prepared simply to illustrate the pattern of hippocampal surface deformation identified by high-dimensional brain mapping and do not provide tests of group differences in any specific subregion.
The relationships of hippocampal shape, hippocampal volume, and total brain volume to clinical and demographic variables were examined. Correlation was used for continuous clinical and demographic variables, and t tests were used for dichotomous variables.
Results
The study participants were 27 patients with major depressive disorder and 42 healthy comparison subjects. The two groups were similar in gender distribution (15 women and 12 men among the patients and 23 women and 19 men among the comparison subjects) and age (mean=33.0 years, SD=10.7, in the patients and mean=33.2 years, SD=10.8, in the comparison subjects). Of the comparison subjects, 39 were right-handed, two were left-handed, and one was ambidextrous. Of the patients, 18 were right-handed, and one was left-handed; handedness was not known in eight cases. In the cases where handedness was not known, this variable had not been assessed during the initial evaluation and it was not possible to locate the subjects subsequently in order to determine handedness. The depressed patients had mean scores of 27.3 (SD=5.2) on the Hamilton depression scale, 8.4 (SD=2.4) on the Core Endogenomorphic Scale, and 4.3 (SD=0.7) on the CGI, suggestive of moderately severe depression. The mean length of the current depressive episode was 59.4 months (SD=115.1), but the median was only 10.0 months, reflecting a skewed distribution. The mean number of previous depressive episodes was 0.8 (SD=1.2), and the mean lifetime number of months spent in depressive episodes was 98.8 (SD=143.4). Fourteen depressed patients were currently receiving psychiatric medications, and 13 were not.
The patients and comparison subjects did not differ significantly in the volume of the left hippocampus (mean=2546.0 mm3, SD=392.7, in the patients and mean=2475.0 mm3, SD=359.4, in the comparison subjects) (F=0.6, df=1, 67, p=0.44) or the volume of the right hippocampus (mean=2948.4 mm3, SD=446.7, in the patients and mean=2993.9 mm3, SD=414.2, in the comparison subjects) (F=0.2, df=1, 67, p=0.67). The total brain volumes were also similar in the depressed patients (mean=1,002,733 mm3, SD=130,031) and comparison subjects (mean=992,789 mm3, SD=105,830) (F=0.1, df=1, 67, p=0.73).
The 10 components representing hippocampal shape accounted for 79.5% of the variance in the complete set of transformation vectors. We found highly significant differences between the depressed patients and the healthy comparison subjects on these 10 components together (Wilks’s lambda=0.145, F=34.1, df=10, 58, p<0.001). The mean score on the associated discriminant function (i.e., the linear combination of the 10 components that produced the greatest discrimination between groups) was –2.98 (SD=1.06) for the depressed patients and 1.92 (SD=0.96) for the healthy comparison subjects, and the range of scores was from –5.41 to –1.55 among the patients and from –0.38 to 4.00 among the comparison subjects. Thus, the 10 components representing hippocampal shape collectively produced a complete separation between the patients with major depressive disorder and the comparison subjects.
The difference between the depressed patients and the comparison subjects in hippocampal shape is associated with a specific pattern of deformity on the hippocampal surface (
Figure 1). The anatomical basis of this deformity in the hippocampus can be suggested by comparison with a surface map of the normal hippocampal subfields (
Figure 1). It is difficult to make this comparison in detail, however, because the correspondence between the maps is inexact. In particular, the normal subfield map was generated from a separate healthy subject and may differ from the surface contours used to represent study group differences.
On the normal map, subfield CA1 accounts for much of the hippocampal head and for the lateral and posterior edges of the hippocampal body and tail. This region can be seen in the deformity maps to be relatively free of inward deformation. In contrast, the subiculum accounts for a smaller region in the medial part of the head and body of the hippocampus. This is best seen in the inferior view, because the subiculum is partially covered over by the dentate gyrus and subfields CA3 and CA4 in the superior view. Much of this region shows considerable inward deformation among the depressed patients, although the correspondence is not precise.
It is important to note that, as drawn in
Figure 1, the subiculum includes the parasubiculum and presubiculum. These regions are on the medial aspect of the subiculum, where there is less inward deformation, and they have considerable connectional and other differences from the subiculum proper
(34). It is probable that they would be affected differentially in major depressive disorder. The subiculum proper occupies the strip immediately medial to subfield CA1 on the inferior aspect of the hippocampus, in the region where the inward deformation is greatest.
In the total group of subjects, scores on the discriminant function representing hippocampal shape did not differ between men and women (t=–0.3, df=67, p=0.76). However, the volumes of the left hippocampus (t=–4.1, df=67, p<0.001), right hippocampus (t=–5.5, df=67, p<0.001), and total brain (t=–6.1, df=67, p<0.001) were significantly smaller in women than men. The ANOVAs comparing depressed patients and comparison subjects on the volume measures were then recomputed with gender as a covariate. The assumption of no factor-by-covariate interaction was tested and met. In these revised analyses, as previously, the depressed patients and comparison subjects did not differ significantly on the volume of the left hippocampus (F=0.8, df=1, 66, p=0.38), right hippocampus (F=0.2, df=1, 66, p=0.64), or total brain (F=0.2, df=1, 66, p=0.63).
In the total group of subjects, age did not correlate significantly with the discriminant function (r=0.04, df=67, p=0.78) or with the volume of the left hippocampus (r=–0.13, df=67, p=0.29), right hippocampus (r=–0.08, df=67, p=0.54), or total brain (r=–0.12, df=67, p=0.34). The correlations of age with these variables were similar and nonsignificant when the depressed patients and comparison subjects were examined separately. Within the group of depressed patients, we computed correlations of the Hamilton depression scale score, Core Endogenomorphic Scale score, CGI score, duration of the current depressive episode, number of lifetime depressive episodes, and lifetime number of months spent in depressive episodes with the discriminant function, left hippocampal volume, right hippocampal volume, and total brain volume. Of these correlations, the only one that was statistically significant was a negative correlation between the number of lifetime depressive episodes and total brain volume (r=–0.42, df=25, p=0.03). The discriminant function score and volumes of the left hippocampus, right hippocampus, and total brain did not differ significantly between patients who were receiving psychiatric medication and those were not.
An additional analysis was conducted to address the confound of unknown handedness in eight of the 27 patients with major depressive disorder. The group of 19 patients with known handedness was matched to a subgroup of 30 comparison subjects taken from the total group of 42 healthy comparison subjects. Matching involved identification of a subgroup of comparison subjects having a gender distribution that was similar to the distribution in the patients and having similar ages within each gender to those in the patients. Matching was conducted without knowledge of data other than age and gender. The resulting subgroups consisted of a depressed subgroup with eight women and 11 men and a comparison subgroup of 13 women and 17 men. The mean ages were 33.1 years (SD=11.7) for the patients and 33.2 years (SD=11.7) for the comparison subjects. Of the patients, 18 were right-handed and one was left-handed, while 28 of the comparison subjects were right-handed and two were left-handed. The mean clinical ratings for the patients were 26.6 (SD=5.7) on the Hamilton depression scale, 7.9 (SD=2.4) on the Core Endogenomorphic Scale, and 4.2 (SD=0.7) on the CGI. For the two subgroups together, the transformation vectors computed by high-dimensional brain mapping were subjected to a new principal components analysis, and the first 10 components were taken to represent hippocampal shape. In this subgroup analysis, there were no significant differences between the depressed patients and comparison subjects in the volume of the left hippocampus (F=0.1, df=1, 47, p=0.75), right hippocampus (F=0.4, df=1, 47, p=0.52), or total brain (F=0.0, df=1, 47, p=0.90). However, the MANOVA conducted on the 10 components representing hippocampal shape revealed highly significant differences between the patients and comparison subjects (Wilks’s lambda=0.126, F=26.4, df=10, 38, p<0.001). The mean scores on the discriminant function associated with the MANOVA (i.e., the linear combination of the 10 components that produced the greatest discrimination between groups) were –3.2 (SD=1.0) for the depressed patients and 2.1 (SD=1.0) for the comparison subjects. There was no overlap between the two groups in scores on this discriminant function. Thus, a reanalysis confined to the patients with known handedness produced results similar to those from the analysis of data from the full patient and comparison groups.
In a final analysis we examined data exclusively from the right-handed patients in the two subgroups (18 depressed patients and 28 comparison subjects). In this analysis, there were no group differences in the volume of the left hippocampus (F=0.1, df=1, 44, p=0.73), right hippocampus (F=0.3, df=1, 44, p=0.59), or total brain (F=0.0, df=1, 44, p=0.97). Again, MANOVA conducted on the 10 components representing shape revealed highly significant group differences (Wilks’s lambda=0.112, F=27.7, df=10, 35, p<0.001). On the associated discriminant function, there was again no group overlap; the values of all of the patients with major depressive disorder were lower than those for all of the comparison subjects.
Discussion
In this study, patients with major depressive disorder did not differ from healthy comparison subjects in hippocampal volume but did differ markedly from them in the shape of this brain structure. The depressed patients and comparison subjects had no overlap on the discriminant function that was a linear combination of the shape indexes. The indexes of hippocampal shape were not associated with other clinical variables, although there was a negative correlation between the lifetime number of depressive episodes and total brain volume.
The results of this study differ from the results of studies that have shown significantly smaller hippocampal volume in depressed patients than in comparison subjects
(2,
5–7). However, in several other studies
(11–
13) the specific volume of the hippocampus was measured and showed no difference between depressed patients and comparison subjects. The differences in the results of these studies may stem from different populations of depressed patients. As already discussed, all of the studies that showed smaller hippocampal volume involved patients with highly recurrent or treatment-resistant depression, while none of the negative findings came from studies in which specific hippocampal volumes were measured and patients had these types of depression. The patients in the study reported here had an average of less than one prior depressive episode and were not selected for treatment resistance. Thus, the findings reported here with regard to hippocampal volume are consistent with the general pattern in the literature.
The negative correlation between the number of lifetime depressive episodes and total brain volume bears some similarity to the finding by Sheline et al.
(2,
35) that, among patients with major depressive disorder, the lifetime duration of depression correlated negatively with hippocampal volume. Sheline et al. did not report correlations between duration of depression and total brain volume. Together, the data suggest that depressive recurrences may be associated with degenerative changes in the brain, so that patients who have experienced multiple episodes may have overall volume reductions, while patients who have had relatively few episodes of major depressive disorder may not show discernible volume effects. Patients with relatively nonrecurrent depression may have separate abnormalities in hippocampal structure that are detectable by shape analysis. Whether the latter structural abnormalities are related to degenerative changes that may be associated with depressive recurrence is not clear.
While we found differences between patients with major depressive disorder and healthy comparison subjects in indexes of hippocampal shape, characterizing this statistical finding in terms of hippocampal topography is a matter of interpretation. Inspection of the surface maps that represent differences between the depressed patients and comparison subjects suggests that the shape abnormality in the patients corresponds in part to inward deformation of the hippocampal surface overlying the subiculum. However, this study has not provided a test of group differences involving any particular subregion. In principle, high-dimensional brain mapping could be applied to characterize the shape of hippocampal subfields, but the necessary methods have not yet been developed. It is noteworthy that the pattern of surface deformities associated with major depressive disorder appears different from the pattern identified among schizophrenia patients in previous research using high-dimensional brain mapping
(20,
21). The latter studies, like the one reported here, did not show differences between patients and comparison subjects in hippocampal volume, but they did identify group differences in hippocampal shape. However, inspection of surface maps suggested that the shape abnormality in the schizophrenia patients was localized to regions of the hippocampal head that were relatively spared among the depressed patients in this study.
A structural abnormality in the subiculum could have implications for the pathophysiology of depression. The subiculum receives inputs from CA1 and other hippocampal subfields, and it is the origin for many of the outputs from the hippocampus, including projections to the ventromedial prefrontal cortex, thalamus, hypothalamus, and striatum. There are also substantial axonal connections with other limbic structures, such as the amygdala and entorhinal cortex
(34). An abnormality in the subiculum could have widespread effects throughout these circuits. Functional imaging studies
(36–
38) have suggested that depression is a circuit disorder involving the amygdala, medial thalamus, ventromedial prefrontal cortex, and other structures related to the subiculum. A postmortem study
(39) indicated that neurons in the subiculum had low spine density and axonal arborization on apical dendrites among a small group of patients with mood disorders. Another study
(40) provided evidence of apoptosis in the subiculum in patients with major depressive disorder. There is a need for further characterization of structural and functional abnormalities in the subiculum and other related structures in subjects with mood disorder.
Limitations of this study should be noted. The study groups were relatively small, and since this study is, to our knowledge, the first to identify specific abnormalities of hippocampal shape in patients with major depressive disorder, replication with a larger number of subjects is particularly important. In addition, the patients were studied during an acute episode of depression, and this study does not provide information regarding whether the identified structural abnormalities are reversible concomitants of an acute episode or enduring characteristics that do not depend on acute symptoms. Making this distinction could have important implications for pathophysiology.