Recent glutamatergic models of schizophrenia have suggested the possible role of glutamate-induced neurodegeneration in schizophrenia
(1,
2). Regions implicated by those models include the anterior cingulate and thalamus
(2) (and parts of the limbic system), areas that are connected via glutamatergic neurons
(3). In vivo levels of glutamate, glutamine,
N-acetylaspartate, total creatine, choline-containing compounds,
scyllo-inositol, and
myo-inositol can be measured simultaneously by using short-echo
1H magnetic resonance spectroscopy (MRS)
(4). In our previous 4.0-T
1H MRS study
(5), we found increased levels of glutamine and normal levels of
N-acetylaspartate in the left anterior cingulate and thalamic regions of never-treated, first-episode patients with schizophrenia compared with healthy volunteers. In this study, we report the first short-echo-time
1H MRS findings at 4.0 Tesla for 1.5-cc volumes of the left anterior cingulate and thalamus in chronically ill, medicated patients with schizophrenia. We hypothesized that relative to healthy volunteers, chronically ill, medicated patients with schizophrenia would have decreased levels of glutamatergic metabolites and
N-acetylaspartate and that levels of glutamatergic metabolites and
N-acetylaspartate would correlate negatively with duration of positive symptoms if there is a neurodegenerative process in these patients.
Method
Twenty-one outpatients with chronic schizophrenia (20 men and one woman) and 21 healthy volunteers (20 men and one woman) participated in the study after giving informed written consent according to the guidelines of the Review Board for Health Sciences Research Involving Human Subjects at the University of Western Ontario. The mean age of the 21 patients was 37 years (SD=11). The mean age of the healthy subjects was 33 years (SD=12).
All patients and comparison subjects were assessed as in our previous study
(5). The Structured Clinical Interview for DSM-IV (SCID) was administered by two interviewers who arrived at a consensual diagnosis. Twelve patients were classified as having paranoid schizophrenia, three as having undifferentiated schizophrenia, and six as having residual schizophrenia. The patients’ mean total scores on the Scale for the Assessment of Negative Symptoms and the Scale for the Assessment of Positive Symptoms were 37 (SD=8) and 18 (SD=17), respectively. An average of 188 months (SD=107) had elapsed since the first positive symptoms had emerged in the patients. The average chlorpromazine equivalent dose of medication
(6) was 436 mg (SD=354). Thirteen patients were receiving treatment with conventional neuroleptics alone. One patient was receiving both a conventional antipsychotic medication and clozapine, while the remaining patients were being treated with risperidone, quetiapine, ziprasidone, or clozapine alone. In addition to the antipsychotic medication, 10 patients were receiving an anticholinergic agent, and four patients were receiving a benzodiazepine. The mean parental education of the highest educated parent rated on a 4-point scale was 1.9 (SD=1.0) for the patients and 2.6 (SD=1.1) for the healthy subjects. All subjects were right-handed with the exception of four patients and four healthy volunteers, who were rated left-handed or ambidextrous on a handedness questionnaire. None of the patients or volunteers had a history of head injury, drug or alcohol abuse in the year before the scan, or serious medical illnesses according to information provided during the SCID.
In vivo short-echo
1H-stimulated echo acquisition mode spectra were obtained from 1.5-cc voxels in the left anterior cingulate and thalamus by using a Varian/Siemens Unity Inova 4.0 Tesla system (TR=2000 msec, TE=20 msec, TM=30 msec, dwell time=500 μsec, 256 averages). Lineshape-corrected and water-subtracted spectra were fitted in the time domain by using a priori knowledge from 12 metabolite solutions in a constrained Levenberg-Marquardt minimization algorithm. Metabolite levels were normalized to the amplitude of a water-unsuppressed acquisition (16 averages). Data acquisition and processing have been described in detail in our previous publications
(4,
5). As in those earlier reports, the amplitude of the unsuppressed acquisition was corrected for the relative gray matter, white matter, and CSF content of the voxel
(7). Metabolites (excluding macromolecules) with coefficients of variation less than 75% were compared between groups with region-wise multivariate analysis of variance and constituent univariate analyses of variance. One-tailed statistics were used for levels of
N-acetylaspartate, glutamate, and glutamine because of the directional hypothesis. Two-tailed statistics were used for other metabolites, with a significance level of p<0.02 in the anterior cingulate and p<0.01 in the thalamus used to preserve a region-wise Bonferroni-adjusted alpha of 0.05. The quantification precision (interindividual coefficient of variation) of
N-acetylaspartate, glutamate, and glutamine concentration measurements has been previously reported to be approximately 8%, 11%, and 24%, respectively, using this technique
(4). Correlations between
N-acetylaspartate, glutamate, glutamine, and duration of illness in patients (from the onset of positive symptoms) and age in healthy subjects were evaluated with the Pearson product-moment correlation coefficient.
Results
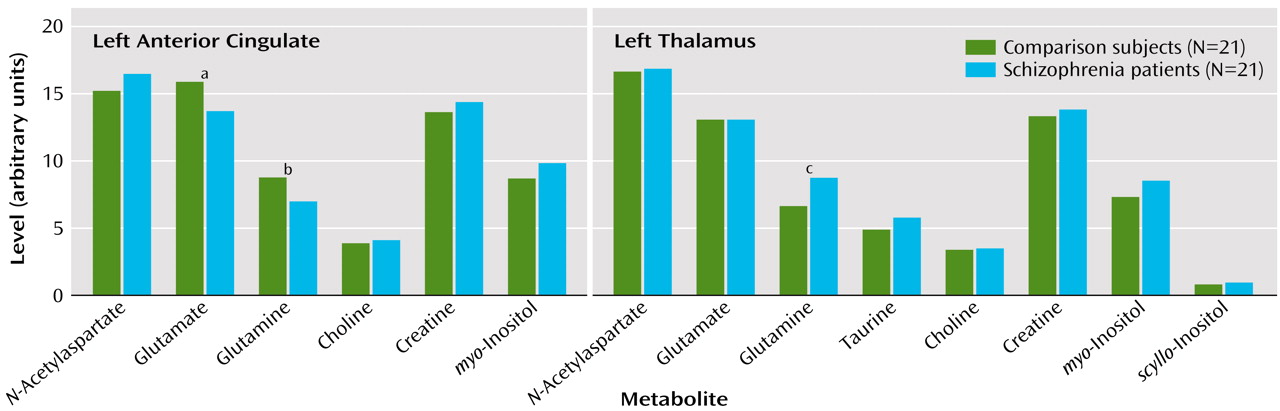
The multivariate analysis of variance comparing all metabolites was significant only in the left anterior cingulate (F=2.58, df=6, 35, p<0.04). Mean metabolite levels for both regions studied as well as the significant between-group differences in individual metabolite levels are presented in
Figure 1. The left thalamus glutamine result, although opposite to our hypothesis, was significant according to two-tailed statistics. Despite the small study group size, a significant difference was found between patients receiving typical (N=13) versus atypical (N=7) antipsychotic medication: a higher level of choline-containing compounds was seen in the left thalamus of patients receiving atypical medication (F=5.69, df=1, 18, p=0.03). Significant correlations were found in the left thalamus between
N-acetylaspartate level and duration of illness in patients (r=–0.47, df=19, p<0.05), between choline-containing compounds level and age in patients (r=–0.52, df=19, p<0.03), and between
myo-inositol level and age in healthy volunteers (r=0.56, df=19, p<0.02). There was no correlation between
N-acetylaspartate levels and age for the two regions studied in either healthy volunteers or patients.
Discussion
The decrease in both glutamate and glutamine in the anterior cingulate contrasts with our earlier finding of an increase in glutamine in first-episode, never-treated schizophrenia patients. Released glutamate is taken up by astrocytes where it is converted to glutamine and transported back to the presynaptic neuron where it is reconverted to glutamate
(8). A decrease in both glutamate and glutamine in the left anterior cingulate could reflect a decrease in the number of glutamatergic synapses in this region in patients with chronic schizophrenia. Although this would be expected with a neurodegenerative process, it could also reflect atrophy or the effects of medication. We have not observed decreases in glutamatergic metabolites assessed with the same methods in first-episode patients treated with neuroleptics for an average of 9 months
(9), so the effects of medication seem less likely. The finding of increased glutamine in the thalamus was the same as that found in our earlier study of never-treated patients. This likely indicates that increased glutamatergic activity in the thalamus persists as the illness progresses but could also be related to medication.
We did not observe decreased
N-acetylaspartate in either region in chronic medicated patients in contrast to several recent studies
(10–
14). Some differences from previous studies could be explained by our correction of the amplitude of the water signal for the voxel content of gray matter/white matter versus CSF and our normalization of metabolite amplitudes to the corrected water signal amplitude or our use of a short-echo-time, single-voxel acquisition technique. However, we did observe a correlation between
N-acetylaspartate and duration of illness that could be related to a neurodegenerative process. Longitudinal studies in drug-naive patients could help clarify the role of medications, acute symptoms, and disease process by examining changes after stabilization with medication and after 2–3 years of illness.