Binge eating disorder is characterized by recurrent episodes of binge eating without the compensatory weight loss behaviors of bulimia nervosa or anorexia nervosa
(1–
3). It occurs in 1.5%–2% of the general population
(1–
4). One way in which binge eating disorder differs from bulimia nervosa and anorexia nervosa is in its association with obesity
(1–
5). People seeking treatment for binge eating disorder are often overweight or obese
(1–
3). Conversely, binge eating disorder is common among obese individuals seeking weight management, occurring in approximately 8%–19% of obese patients in weight loss programs, 70% of individuals in Overeaters Anonymous, and 25% of bariatric surgery patients
(1–
3,
5,
6).
There is no established ideal treatment for binge eating disorder, particularly when it is associated with obesity
(2). Although
d-fenfluramine
(7) (a serotonin-enhancing appetite suppressant that has been removed from the market), selective serotonin reuptake inhibitors (SSRIs)
(8–
10), tricyclic antidepressants
(11,
12), cognitive behavior therapy (2, 3, 13), interpersonal therapy
(2,
3,
13), and behavioral dietary treatment
(2,
3) have all been shown in controlled studies to be effective in decreasing binge eating in binge eating disorder and related conditions, their effects on associated obesity have been variable
(2). In general, SSRIs
(8–
10) and imipramine
(12)—but not
d-fenfluramine
(7), desipramine
(11), cognitive behavior therapy
(2,
3,
13), interpersonal therapy
(2,
3,
13), or behavioral dietary treatment
(2,
3)—have been associated with weight loss. Degree of weight loss, however, has been modest
(8–
10,
12).
Topiramate is a structurally novel agent approved in many countries for the treatment of epilepsy
(14). Its pharmacologic mechanisms involve modulation of voltage-dependent sodium and calcium channels, enhancement of γ-aminobutyric acid (GABA) activity at a nonbenzodiazepine site on GABA
A receptors, and blockade of kainate/AMPA glutamate receptors
(14).
Several lines of evidence suggest that topiramate might be effective in the treatment of binge eating disorder. First, we have observed improvement of co-occurring binge eating disorder in patients receiving topiramate for treatment of mood disorders
(15). Second, topiramate was associated with anorexia and weight loss in clinical trials with epilepsy patients
(14,
16). Third, animal studies have shown that stimulation of the lateral hypothalamus by glutamate and glutamate agonists, including kainate/AMPA agonists, causes an intense, rapid, dose-dependent increase in food intake
(17). Topiramate, as noted, is an antagonist at kainate/AMPA glutamate receptors.
We therefore conducted a single-center, randomized, parallel-group, placebo-controlled study to assess the efficacy and safety of topiramate during a 14-week course of treatment in 61 outpatients with binge eating disorder associated with obesity.
Method
Patients
Study participants were outpatients at the University of Cincinnati Medical Center who were between 18 and 60 years of age, met DSM-IV-TR criteria for binge eating disorder, were obese (defined as having a body mass index ≥30 kg/m
2)
(3,
5), and, to ensure they had marked distress regarding their binge eating, had a score ≥15 on the Yale-Brown Obsessive Compulsive Scale
(18), which was modified for binge eating (available from the first author upon request). DSM-IV-TR criteria for binge eating disorder are recurrent episodes of binge eating associated with at least three of five indicators of impaired control and marked distress but without the regular use of inappropriate compensatory behaviors. Also, the binges must occur at least 2 days a week for 6 months. The modified Yale-Brown Obsessive Compulsive Scale measured obsessiveness of binge eating thoughts and compulsiveness of binge eating behaviors. Patients were recruited by a radio advertisement requesting volunteers for a study of a medication for the treatment of binge eating associated with obesity.
We excluded patients from participation if any of the following were present or occurred: 1) substance use disorder (per DSM-IV-TR) within the past 6 months; 2) unstable bipolar disorder (per DSM-IV-TR) within the past 3 months; 3) clinically significant suicidality; 4) any current or past psychiatric disorder that could interfere with diagnostic assessment, treatment, or study adherence; 5) clinically unstable medical illness; 6) a history of nephrolithiasis or seizures (including childhood febrile seizures); 7) clinically significant abnormal laboratory results; 8) need for treatment with any medication that might adversely interact with or obscure the action of topiramate (e.g., stimulants, antidepressants, or carbonic anhydrase inhibitors); 9) treatment with psychoactive medications (other than mood stabilizers or zolpidem) within 2 weeks of random assignment; 10) treatment with an experimental drug or an experimental device within 30 days of random assignment; or 11) previous treatment with topiramate.
The study protocol was approved by the University of Cincinnati Medical Center Institutional Review Board, and the study was conducted in compliance with the Declaration of Helsinki. All patients signed approved informed consent forms after the study procedures had been fully explained. Patients were enrolled from September 2, 1998, through June 4, 2000.
Study Design
This was a randomized, parallel-group, placebo-controlled, double-blind, flexible-dose (25–600 mg/day) topiramate trial conducted at the University of Cincinnati Medical Center. The trial consisted of a 2- to 5-week screening period, a 14-week treatment period, and a 2-week treatment taper and discontinuation period. Patients were evaluated at least twice (initial screening examination and baseline assessment) during the screening period; after 1, 2, 4, 6, 8, 10, and 14 weeks during the treatment period; and after weeks 15 and 16 during the 2-week treatment taper and discontinuation period.
The following were obtained or conducted at the initial screening visit: written informed consent; assessment with the Structured Clinical Interview for DSM-IV
(19) (to determine whether the patient met DSM-IV criteria for binge eating disorder and any comorbid axis I psychiatric disorders); the number of binges and binge days during the previous week; psychiatric and medical history; a physical examination; vital sign monitoring; routine blood chemical and hematological tests; fasting blood levels of glucose, insulin, and lipids; an electrocardiogram; and a urinalysis. Patients were given take-home diaries at this and each of the following visits in which to record, on a daily basis, any binges and, once study medication was initiated, the number of medication tablets taken.
At the last visit of the screening period (the baseline assessment), patients were evaluated to see if they continued to meet entry criteria. Patients continuing to meet these criteria were enrolled in the treatment period and randomly assigned in a 1:1 ratio to therapy with topiramate or placebo. All study medication was in identical 25- and 100-mg tablets supplied in numbered containers and dispensed to patients according to a predetermined randomization schedule (see statistical analysis). The initial dose was 25 mg each evening for a minimum of 3 days. Beginning on day 4, the dose was increased to 50 mg each evening. Beginning on day 7, the dose was increased to 75 or 100 mg each evening. If after 2 weeks there was no response (defined as a 50% or greater reduction in binge frequency), the dose was increased at a maximum of 50 mg/week for 4 weeks to a maximum dose of 300 mg/day at 6 weeks. Subsequently, the dose was increased at a maximum of 75 mg/week for 4 weeks to a maximum dose of 600 mg/day at 10 weeks. Study medication dose was not changed from treatment period weeks 10 through 14 unless a medical reason (e.g., an adverse event) required such a change. If a patient did not tolerate any dose increase, the dose could be decreased to one that was tolerable. Study medication was provided in prepackaged coded plastic bottles containing 60 identical 25- or 100-mg topiramate or placebo tablets.
Efficacy Measures
In addition to the assessment of medication dose, medication compliance (through diary review and tablet count), adverse events, use of any nonstudy medications, weight, and vital signs, efficacy measures were assessed at each subsequent visit. The primary efficacy measure was the number of binge eating episodes (binge frequency) during the 7 days before each visit. A binge was defined according to DSM-IV-TR criteria. Binges were assessed by clinical interview and review of patient take-home diaries, in which patients recorded binges, duration of binges, and food consumed during binges (so that binges could be confirmed by the investigator). A secondary measure of efficacy was the number of binge days (binge day frequency) during the 7 days before each visit. A binge day was defined as a day on which a patient had at least one binge eating episode. For visits that occurred less than 7 days after the previous visit, the number of binges and binge days were normalized to a weekly frequency. Other secondary efficacy measures included scores on the Clinical Global Impression (CGI) improvement and severity scales, the modified Yale-Brown Obsessive Compulsive Scale, and the Hamilton Depression Rating Scale
(20) as well as body mass index, weight, waist-to-hip ratio, and percent and total body fat as measured by bioelectrical impedance (Health Management System 1000, Bio/Analogics, Beaverton, Ore.). The CGI improvement scale is a 7-point scale on which 1=very much improved, 2=much improved, 3=minimally improved, 4=no change, 5=minimally worse, 6=much worse, and 7=very much worse. The CGI severity scale is a 7-point scale on which 1=normal, 2=borderline ill, 3=mildly ill, 4=moderately ill, 5=markedly ill, 6=severely ill, and 7=among the most extremely ill. Additional secondary outcome measures included blood pressure and fasting measurements of blood glucose, insulin, and lipids.
At every treatment period visit, we assessed all efficacy measures except for blood tests, waist-to-hip ratio, and percent and total body fat, which were obtained at the week 14 treatment period visit (or the last visit if the patient terminated the study prematurely).
We assessed the following safety measures: adverse events, clinical laboratory data, physical examination findings, and vital signs.
Statistical Analysis
Baseline characteristics of each group were compared by using Fisher’s exact test for categorical variables and the t test for continuous variables.
The primary protocol-defined analysis of efficacy was a repeated-measures random regression analysis comparing the rate of change of binge frequency during the treatment period between groups. The same analysis was applied to binge day frequency, body mass index, weight, and scores on the CGI severity scale, modified Yale-Brown Obsessive Compulsive Scale, and the Hamilton Depression Rating Scale. This analysis was an intent-to-treat analysis and used all observations from all time points from all subjects who completed the baseline visit, even from subjects with only baseline assessments. The difference in rate of change was estimated by random regression methods, as employed in three previous studies of binge eating disorder
(8–
10) and described in Diggle et al.
(21) and Gibbons et al.
(22). We used a model for the mean of the outcome variable that included terms for treatment, time, and the treatment-by-time interaction. We modeled time as a continuous variable, expressed as a log (weeks + 1), with weeks ranging from 0 at baseline to 14 at the week 14 visit after random assignment. The logarithmic transformation was used because the response of the efficacy measures was approximately linear on the log scale, as is often found in treatment studies of psychiatric disorders
(8–
10,
22). For the analyses of binge frequency and binge day frequency, we used the logarithmic transformations log ([binges/week] + 1) and log ([binge days/week] + 1), respectively, to normalize the data and stabilize the variance. The measure of effect was the treatment-by-time interaction, which can be interpreted as the difference in the rate of change (change per unit of time), or the difference in slope with respect to time, of the efficacy measure. To account for the correlation of observations within individuals, we calculated the standard errors of the parameter estimates by using generalized estimating equations, with compound symmetry as the working covariance, as implemented by the PROC GENMOD command in SAS software.
We compared the mean percentage change from baseline in binge frequency and binge day frequency for each treatment group by using the nonparametric Wilcoxon rank sum test for both an intent-to-treat analysis (using the last observation carried forward for each subject with at least one postrandomization assessment) and a completer analysis (patients who completed 14 weeks of treatment). A comparison of the final mean score on the CGI improvement scale in each treatment group was performed by using ANOVA for the same intent-to-treat and completer populations. Response to treatment was also analyzed categorically, with response categories defined by the percentage reduction in binge frequency from baseline: remission=cessation of binges; marked=a 75%–99% reduction; moderate=a 50%–74% reduction; and none=less than a 50% reduction
(8–
10). Treatment group responder comparisons were made by using the exact trend test for 2-by-k ordered tables in SAS.
For laboratory measures, including weight and body fat, we computed the mean difference between endpoint and baseline measures and then compared the treatment groups by using the t test. We calculated the correlation coefficients by using rank-transformed data (Spearman rank correlation). All statistical tests were two-sided with alpha=0.05.
Results
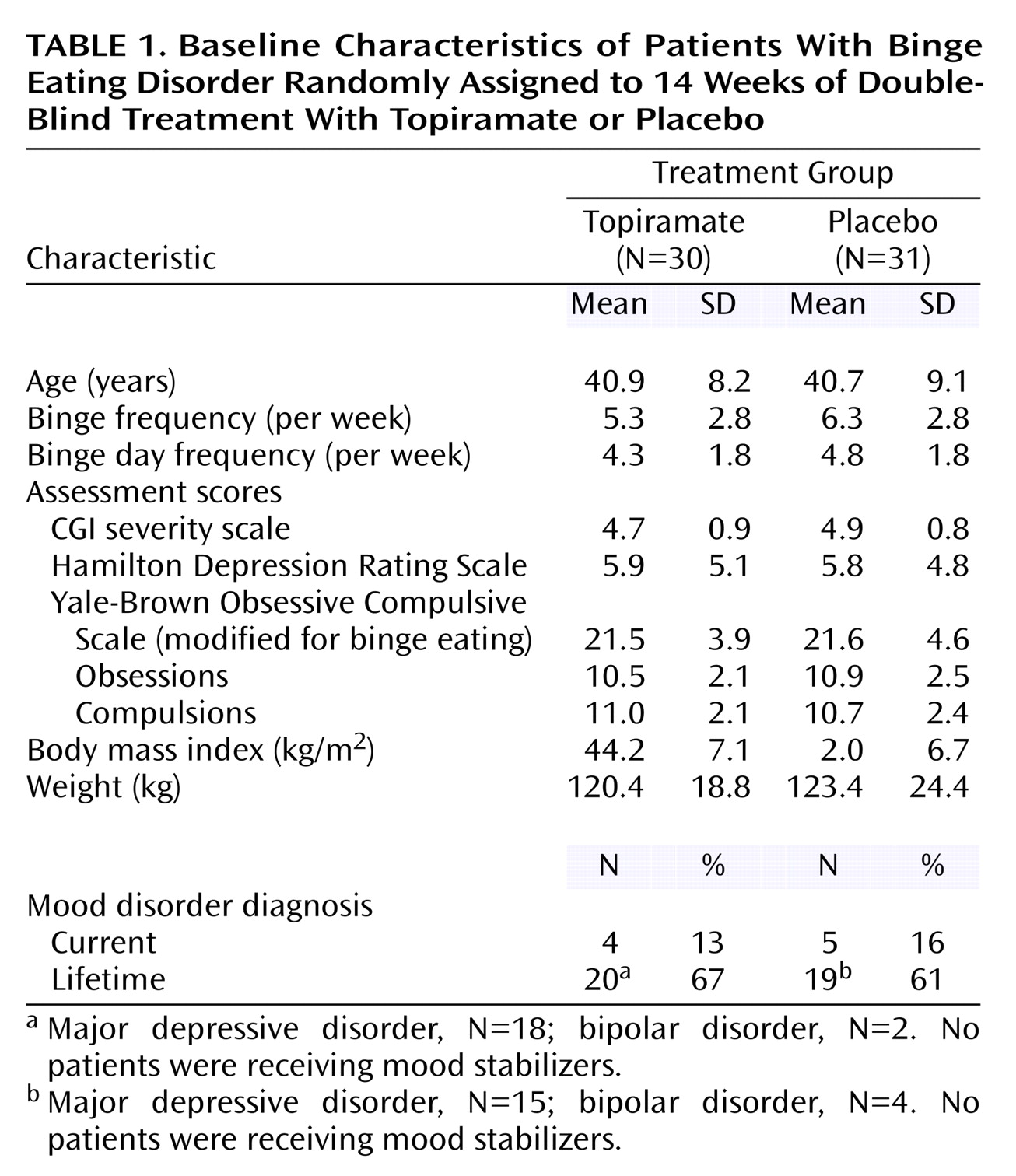
Of 98 subjects screened, 37 were not randomly assigned to a treatment group because they chose not to participate (N=15), had abnormal laboratory values (N=9), did not meet DSM-IV-TR criteria for binge eating disorder (N=6), had exclusionary co-occurring psychiatric disorders (N=4), had unstable medical conditions (N=2), or had a score <15 on the modified Yale-Brown Obsessive Compulsive Scale (N=1). Of the remaining 61 patients, 30 were randomly assigned to topiramate, and 31 were assigned to placebo. At baseline, there were no significant differences between the treatment groups in demographic or clinical features (
Table 1).
Fifty-eight patients (28 receiving topiramate, 30 given placebo) had at least one postrandomization efficacy measure. Fourteen of 30 patients in the topiramate group (46.7%) and 12 of 31 patients in the placebo group (38.7%) did not complete all 14 weeks of treatment. Nine patients withdrew from the study because of adverse events (topiramate: N=6; placebo: N=3), three because of lack of efficacy (topiramate: N=1; placebo: N=2), 13 because of nonadherence with the study protocol (topiramate: N=6; placebo: N=7), and one subject (receiving topiramate) because of an exacerbation of a previous medical condition. The adverse events that caused discontinuation among the topiramate-treated patients were headache (N=3), paresthesias (N=2), and amenorrhea (N=1); leg cramps, sedation, and testicular soreness were the adverse events that caused discontinuation among the patients given placebo (N=1 for each).
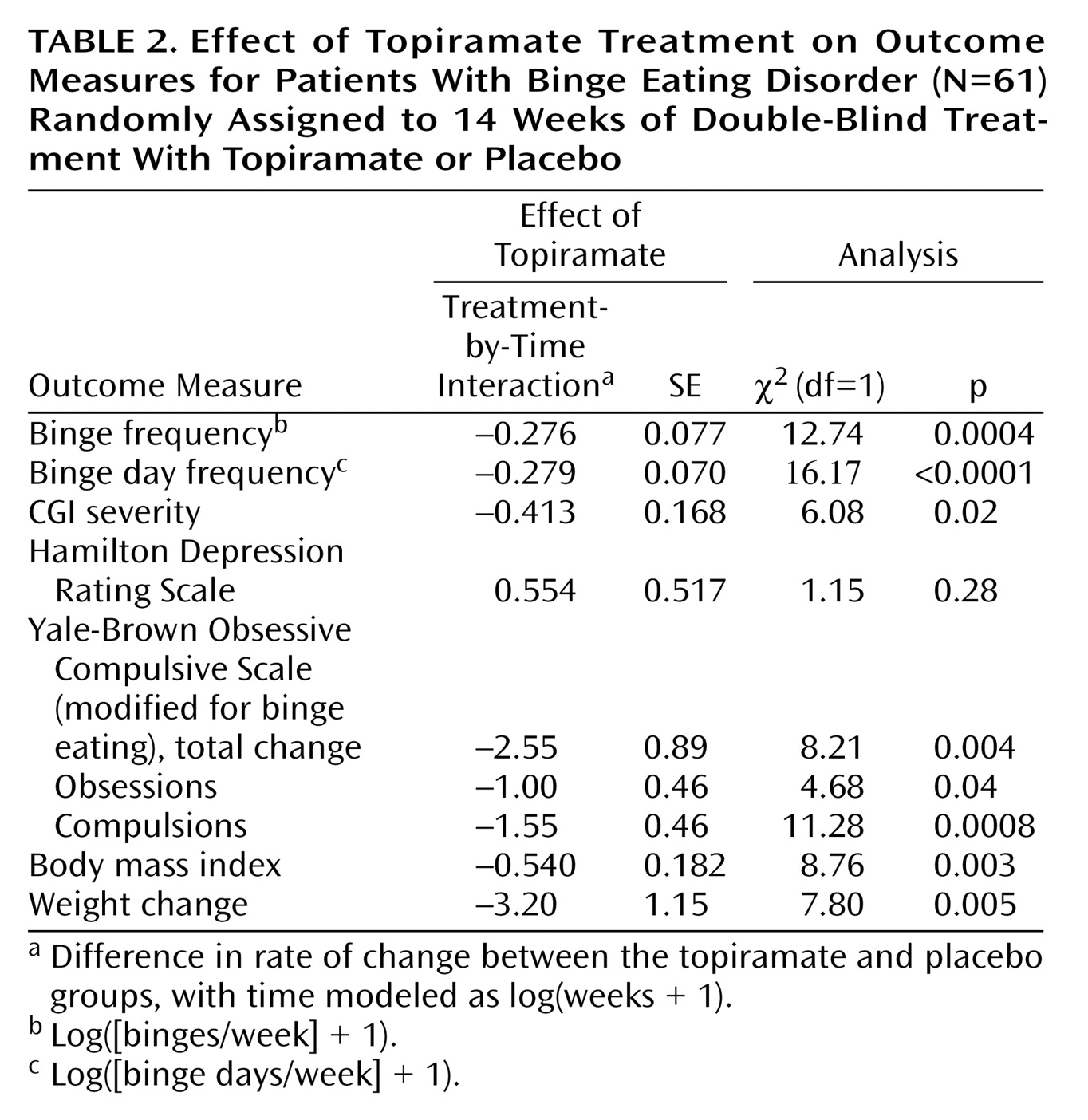
All rates of change data are summarized in
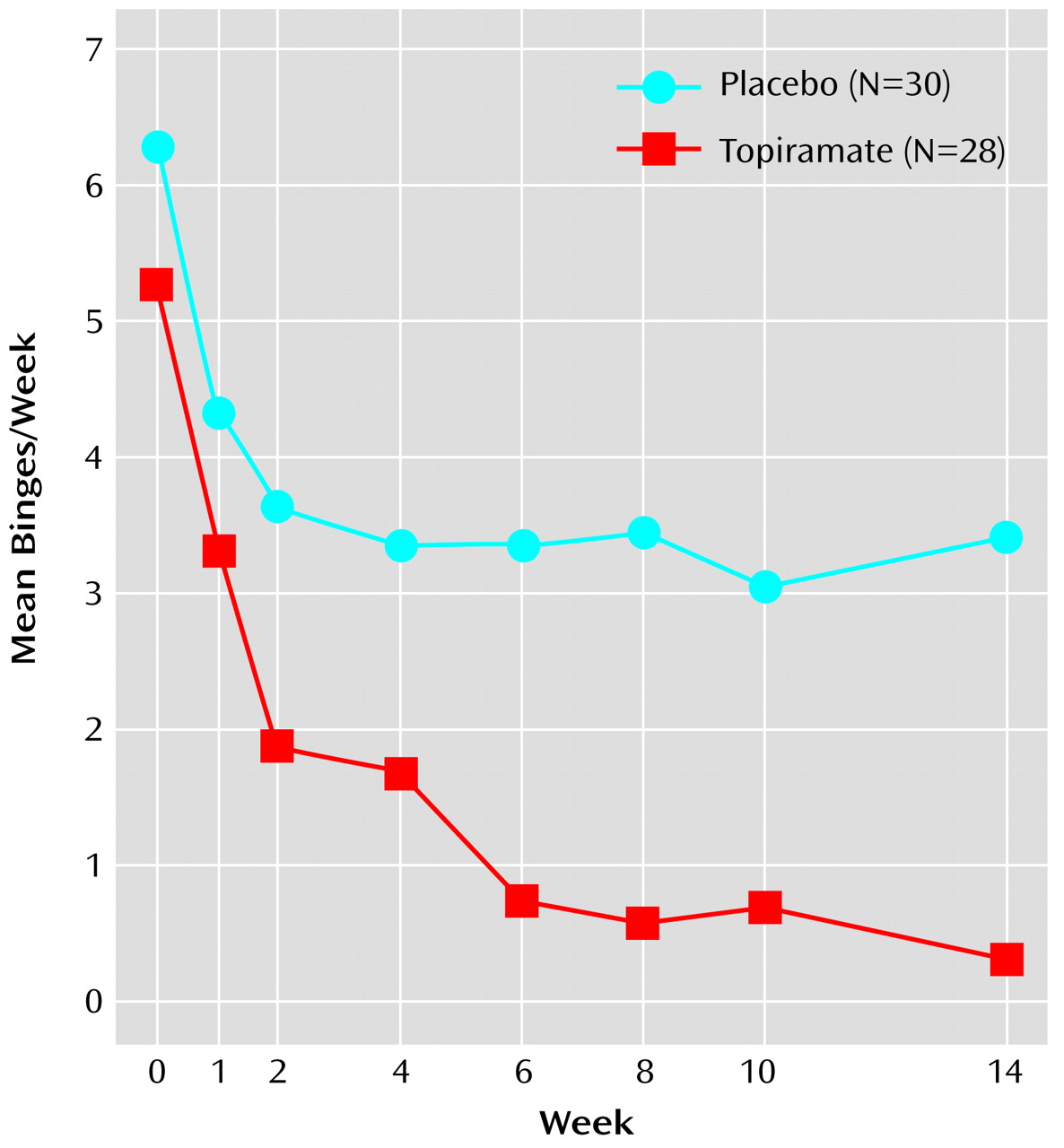
Table 2. The primary analysis of efficacy revealed that topiramate-treated patients had a significantly greater rate of reduction in binge frequency compared with patients given placebo (
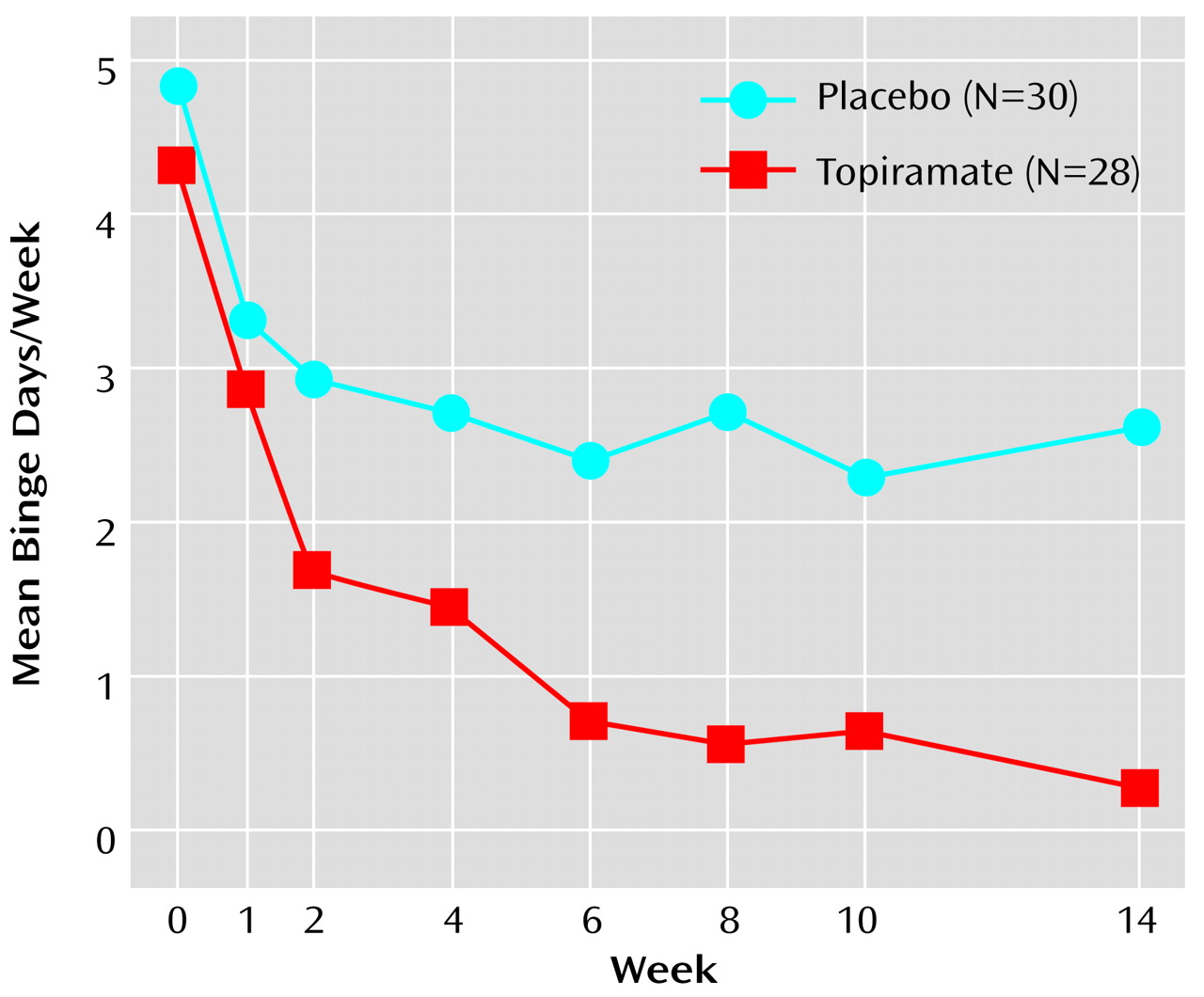
Figure 1). Topiramate was also associated with significantly greater rates of reduction in binge day frequency (
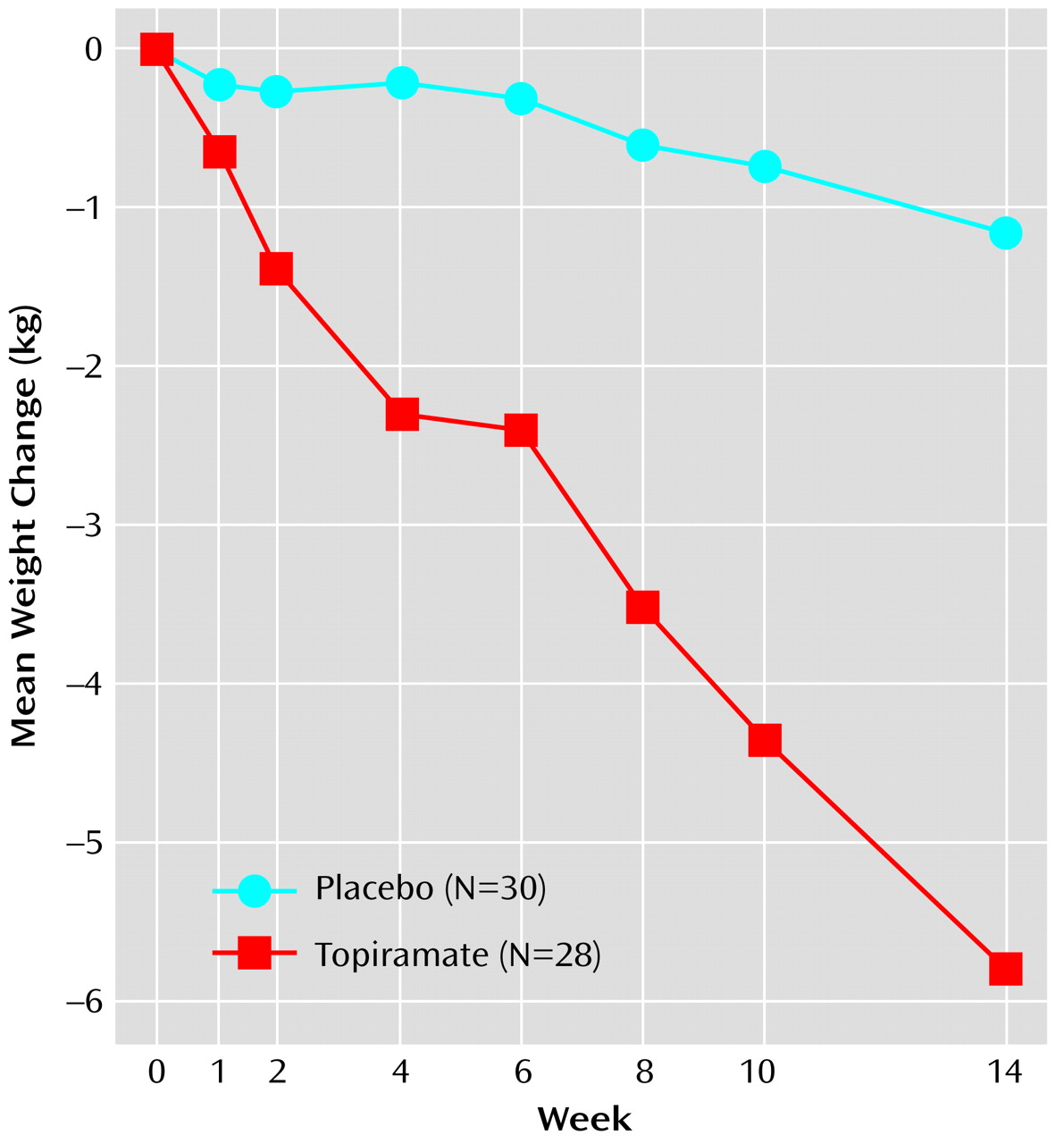
Figure 2), body mass index, weight (
Figure 3), and scores on the CGI severity scale and modified Yale-Brown Obsessive Compulsive Scale. The rate of decrease in Hamilton Depression Rating Scale scores did not differ between treatment groups.
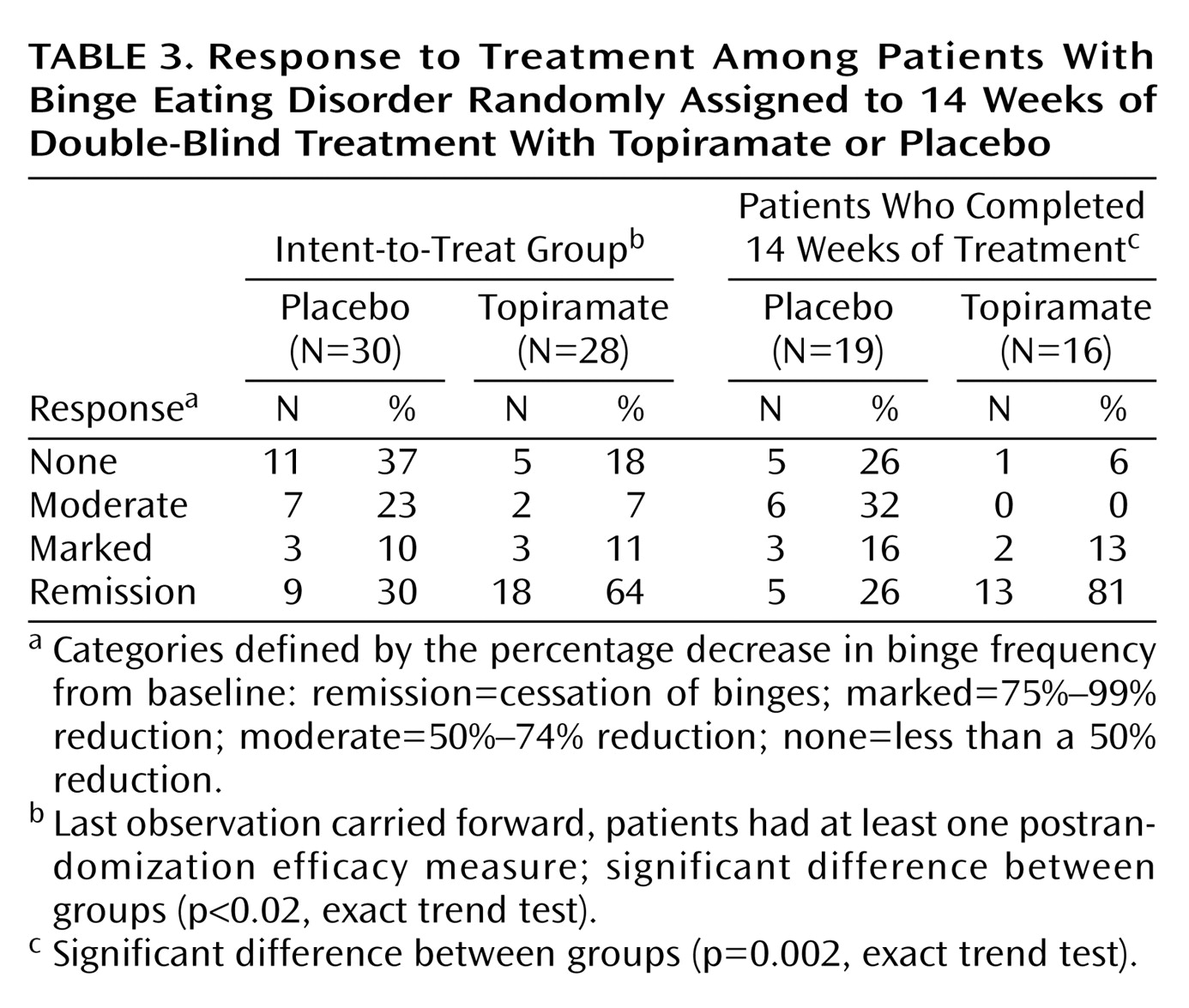
A significantly greater reduction from baseline in binge frequency was seen with topiramate relative to placebo in both the intent-to-treat group (94% versus 46%, respectively; p<0.02, Wilcoxon rank sum test) and in the completer group (94% versus 50%; p=0.002, Wilcoxon rank sum test). Likewise, a significantly greater reduction from baseline in binge day frequency was seen with topiramate relative to placebo in both the intent-to-treat group (93% versus 46%, respectively; p<0.02, Wilcoxon rank sum test) and in the completer group (92% versus 49%; p=0.001, Wilcoxon rank sum test). CGI improvement scores at the last visit were significantly better for patients treated with topiramate in both the intent-to-treat population (χ
2=6.31, df=1, p=0.01) and in patients completing the study (χ
2=5.68, df=1, p=0.02). In the categorical response analyses, there were significantly higher levels of response in the topiramate group compared with the placebo group (
Table 3).
Patients receiving topiramate experienced a mean weight loss of 5.9 kg during the 14-week study, whereas those receiving placebo experienced a mean weight loss of 1.2 kg (
Figure 3). Weight loss since baseline was significantly correlated with reduction in binge frequency at week 14 (r=0.65, df=56, p=0.006). Topiramate-treated patients also had a significant decrease from baseline to week 14 in percent (χ
2=7.87, df=1, p=0.005) and total (χ
2=10.68, df=1, p=0.001) body fat. However, topiramate was not associated with a significantly greater decrease from baseline to week 14 in waist-to-hip ratio than placebo.
Topiramate was associated with a significant change in diastolic blood pressure at the last visit compared with placebo among the intent-to-treat group (–2.71 versus 0.47 mm Hg, respectively; χ2=4.39, df=1, p=0.04) and among patients completing the study (–4.75 versus 0.79 mm Hg; χ2=5.14, df=1, p=0.02). There were no significant differences between patients receiving topiramate and those given placebo in mean change from baseline to final visit for the fasting metabolic measurements of insulin (–5.78 and –0.76 μu/ml, respectively), glucose (–2.4 and –0.82 mg/dl), LDL cholesterol (–8.40 and –0.44 mg/dl), triglycerides (–27.2 and –8.06 mg/dl), and total cholesterol (–17.13 and –2.13 mg/dl).
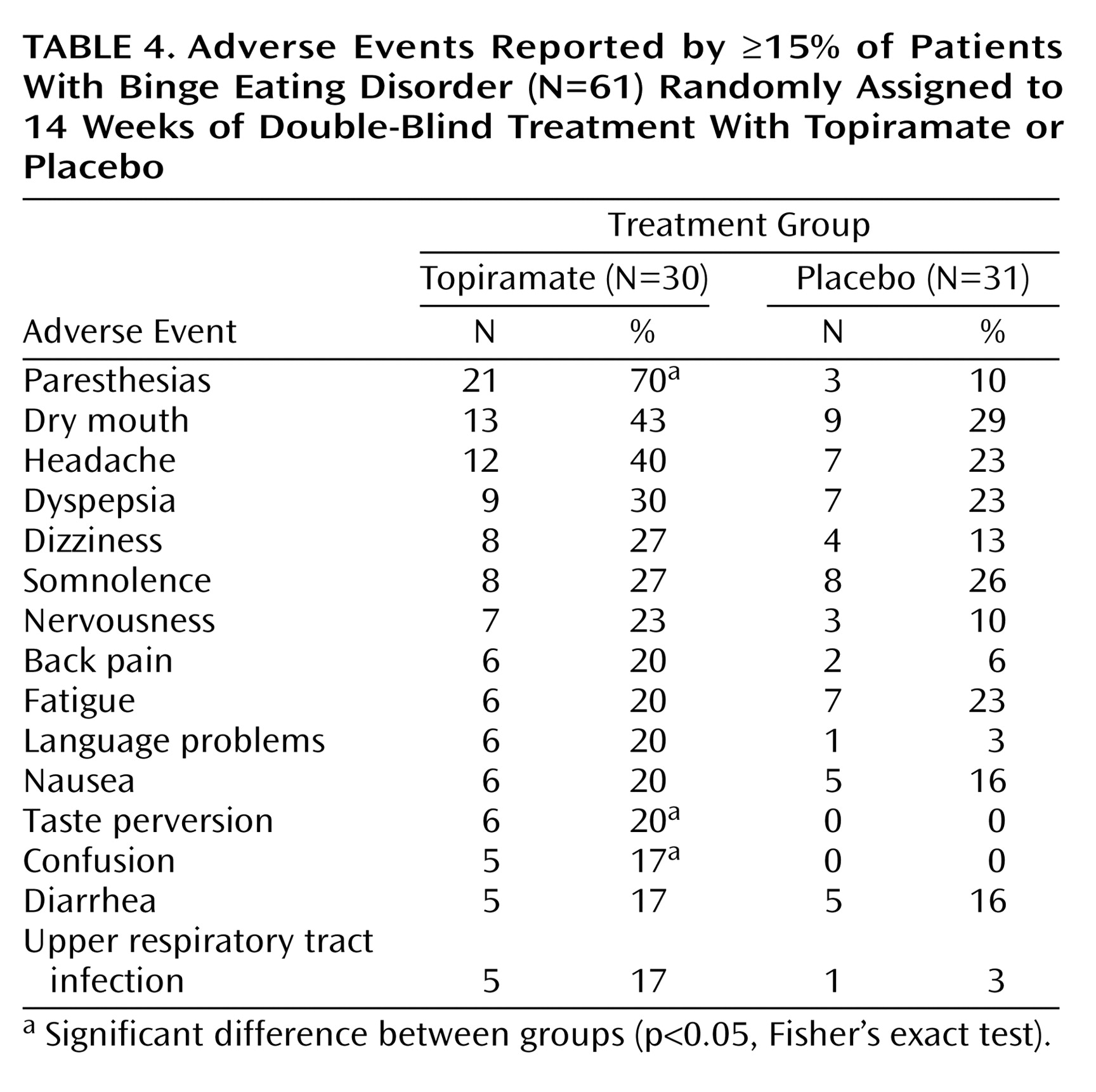
The median dose of topiramate was 212 mg/day (range=50–600), and the median “dose” of placebo was 362 mg/day. Adverse events were more common with topiramate than with placebo but were generally mild or moderate in nature and resolved with time or dose reduction (
Table 4). No serious adverse medical events were observed among the topiramate-treated patients. There were no changes in physical examination findings, vital signs, or clinical laboratory values that suggested drug-related toxicity. There was no evidence of withdrawal symptoms during the taper and discontinuation phase.
Discussion
In this group of individuals with binge eating disorder associated with obesity, a significantly greater rate of reduction in binge frequency was seen with topiramate than with placebo as well as significantly greater rates of reduction in binge day frequency, global severity of illness, obsessive-compulsive features of binge eating symptoms, weight, and body mass index. The mean weight loss in the intent-to-treat group receiving topiramate was 5.9 kg, which was in contrast to the mean weight loss of 1.2 kg in the placebo group. Topiramate was also associated with a significantly higher level of response, including rate of remission, among patients in the intent-to-treat group and those who completed the 14-week treatment period. Among patients who completed the trial, the reduction in binge frequency associated with topiramate correlated with the decrease in weight. Topiramate was also associated with significant improvement in diastolic blood pressure, but laboratory measures of lipids, glucose, and insulin, although improved in those who completed the study, were not significantly different.
Topiramate-treated patients were more likely than patients given placebo to experience adverse events and to withdraw because of adverse events. Nonetheless, topiramate was safe and relatively well tolerated over the 14-week period. We observed no withdrawal symptoms upon drug discontinuation. Also, there were no cases of nephrolithiasis or acute angle glaucoma, rare but serious side effects associated with topiramate
(14,
23).
The reduction in binge frequency and the overall improvement observed with topiramate in this study are at least comparable to the results reported in studies of SSRIs
(8–
10) and
d-fenfluramine
(7) in binge eating disorder, desipramine in nonpurging bulimia nervosa
(11), and imipramine in binge eating associated with obesity
(12). The weight loss effect appears greater for topiramate, but this should be addressed in comparison trials.
The mechanism of action of topiramate in binge eating disorder is unknown. It may act as an appetite suppressant or satiety enhancer and thereby reduce binge eating. The effects of SSRIs and
d-fenfluramine on appetite and binge eating have been hypothesized to be due to their serotonin-enhancing properties
(7–
10). Topiramate has not been shown to affect serotonergic neurotransmission. Indeed, it has been shown to attenuate nicotine-induced increases in mesolimbic extracellular dopamine and norepinephrine, but not serotonin, release
(24). As noted earlier, topiramate antagonizes glutamate receptors
(15,
16). Since glutamate and glutamate agonists rapidly elicit intense eating when injected into the lateral hypothalamus of rats
(17), topiramate might reduce binge eating through glutamate antagonism. Zheng et al.
(25) recently found that injection of the kainate/AMPA receptor antagonist 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfanomide (NBQX) either into the fourth ventricle or directly into the nucleus tractus solitarius potently suppressed food and water intake in rats in a dose-dependent manner. Because alteration of taste, gastrointestinal upset, and nausea are relatively common side effects of topiramate, it is also possible that these effects contributed to the reduction in binge eating in some patients. Nonetheless, preliminary data suggest that the weight loss associated with topiramate in both human obesity and rodent models of obesity is due to loss of fat rather than lean body mass
(16,
26). In the rodent models, topiramate inhibited fat deposition by either reducing food intake or stimulating energy expenditure
(26). The mechanisms by which topiramate affects food intake and energy expenditure remain unknown.
Several limitations of this study should be considered. First, because the duration of double-blind therapy was only 14 weeks, the results may not generalize to longer periods of treatment. However, to our knowledge, this is the longest controlled trial of a medication in binge eating disorder completed to date.
Second, only 35 of the 61 patients randomly assigned to a treatment condition completed the 14-week treatment phase of the study. However, the primary analysis was based on data from all patients randomly assigned to a treatment condition at all time points; furthermore, this analysis would have remained statistically significant when analyzed after 6, 8, and 10 weeks of treatment, with 45, 44, and 39 patients, respectively.
Third, because more topiramate-treated patients experienced adverse events than placebo-treated patients, blindness to treatment assignment may have been compromised in some cases.
In summary, in a placebo-controlled 14-week trial in outpatients with binge eating disorder and obesity, topiramate was superior to placebo on many outcome measures of binge eating behavior and weight and was relatively well tolerated. Topiramate may represent a promising new treatment for binge eating disorder associated with obesity.