The rapid-cycling variant of bipolar disorder has been estimated to occur in 14%–53% of patients
(1–
5). Its prevalence appears to be as low as 4% in bipolar I disorder and as high as 31% in bipolar II disorder in one study
(4). Of these patients, 72%–82% have been reported to exhibit poor response to lithium
(1,
2). Thus, a substantial percentage of poor response to lithium has been associated with rapid cycling.
In an attempt to develop alternative treatments for patients with rapid-cycling bipolar disorder, we previously evaluated the spectrum of acute and prophylactic efficacy of divalproex in 131 patients who received divalproex (either in monotherapy or in combination with other psychotropic drugs) in a prospective, naturalistic, 17-month open-label trial. Sixty percent of these patients were either lithium-resistant or intolerant. The data from this preliminary study suggested that divalproex possessed marked acute and prophylactic antimanic efficacy as well as moderate acute and prophylactic antidepressant efficacy in lithium-naive patients as well as those who had previously not responded to adequate trials of lithium
(6). We hypothesized that divalproex would be more effective than lithium in the long-term treatment of rapid-cycling bipolar disorder and conducted a random assignment, 20-month, double-blind, parallel-group comparison of divalproex and lithium to test this hypothesis.
Method
The study was conducted at the Mood Disorders Program at Case Western Reserve University/University Hospitals of Cleveland between September 1995 and January 2003. Patients could discontinue or be discontinued from any phase of the study for poor tolerance of study medications, lack of medication efficacy, investigator or patient unwillingness to continue the study for any reason, or nonadherence with study procedures. Patient participation in this study could last up to 26.5 months, including a 2-week screening period, a 6-month open-label acute stabilization phase, and a 20-month double-blind, parallel-group maintenance monotherapy phase.
Study Subjects
Patients eligible for participation were men and women, 18 years of age or older, who met DSM-IV criteria for the following, ascertained by clinical interview: 1) bipolar I or bipolar II disorder, 2) rapid cycling during the 12 months preceding study entry, and 3) a history of at least one episode of hypomania, mania, or a mixed state within 3 months of study entry. Patients were required to be in good physical health according to medical history, physical examination, and laboratory analyses (including thyroid function tests) conducted at screening. Patients were excluded from study participation if they had previously taken lithium and divalproex concurrently, experienced intolerable side effects to documented lithium levels of 0.8 meq/liter or valproate levels of 50 μg/ml, were pregnant or planning to become pregnant, were taking exogenous steroids, had met criteria for alcohol or drug abuse or dependence within the preceding 6 months, or were actively suicidal as evidenced by a score ≥3 on that item from the Hamilton Depression Rating Scale
(7). After complete description of the study to the subjects, written informed consent was obtained.
Screening
Screening occurred in the 2 weeks preceding the patient’s entry into the open-label phase. Psychiatric and medical histories were obtained, physical examinations including clinical laboratory tests were performed, scores on psychiatric rating scales (including the 24-item Hamilton depression scale, Young Mania Rating Scale
[8], and the Global Assessment Scale [GAS]
[9]) were obtained, and then a retrospective mood chart was completed over 1–2 months to confirm the existence of four mood episodes in the preceding 12 months
(10). Eligible patients were then enrolled in the open-label acute stabilization phase.
Open-Label Acute Stabilization Phase
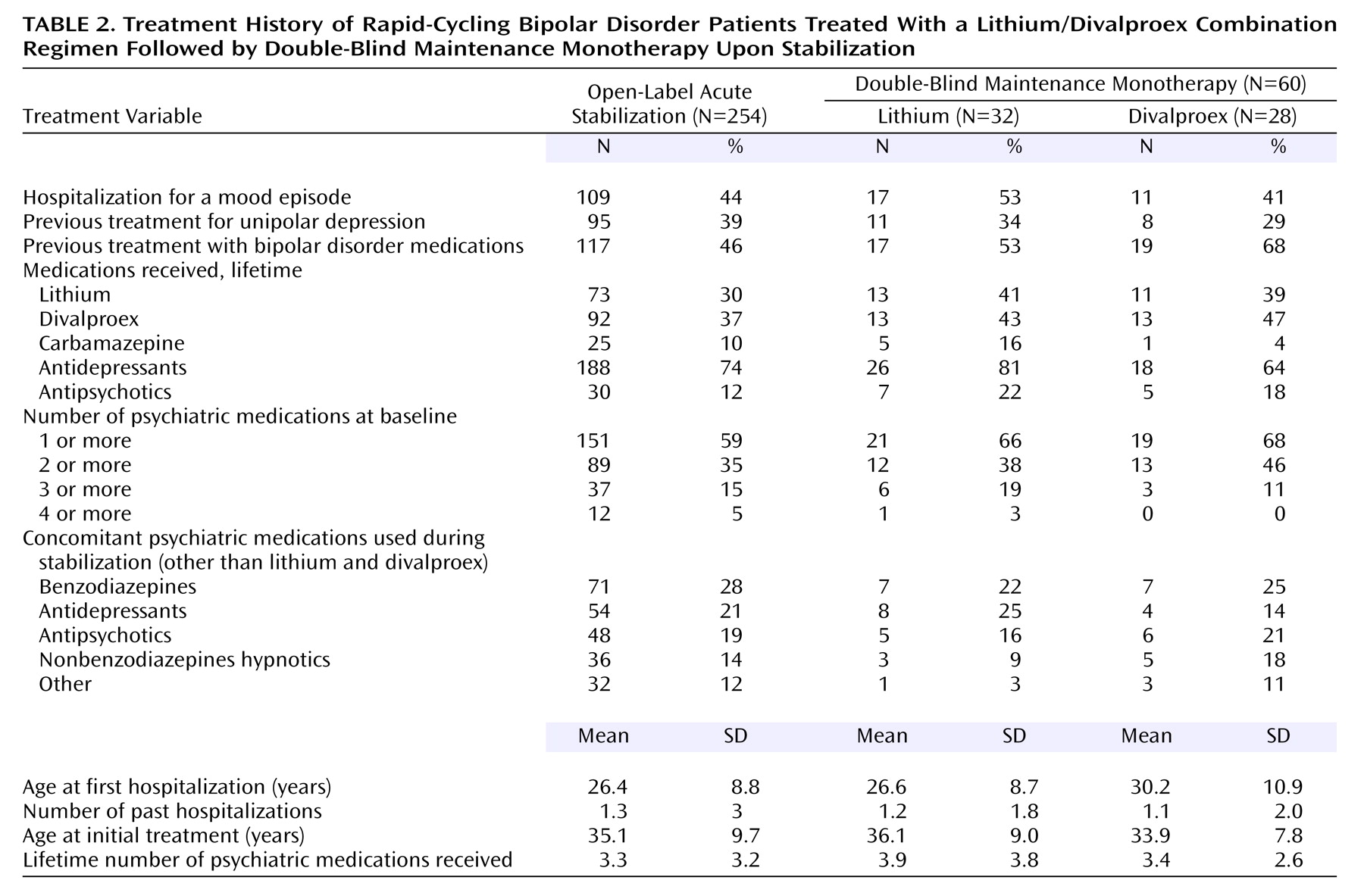
During this phase, patients were seen by a psychiatrist every 2 weeks and treated with the combination of lithium and divalproex sodium. For patients who had been receiving no medication, lithium carbonate monotherapy was initiated at 300 mg twice daily and titrated over 4–6 weeks to minimum blood levels of 0.8 meq/liter. Divalproex augmentation was then initiated at 250 mg twice daily and then increased over 4–6 weeks to minimum blood levels of 50 μg/ml. If patients were already taking psychotropic medications other than lithium and divalproex, these medications were gradually weaned over 3 months as lithium and divalproex were concurrently initiated and titrated as described above. Patients who had previously been treated with lithium or divalproex were allowed into this study as long as they tolerated the medication regimen and had never previously taken both medications concurrently. If patients were already taking lithium, but not divalproex, divalproex was then initiated as described. If patients were already taking divalproex, but not lithium, lithium was then initiated and titrated as described. All psychotropic medications other than lithium and divalproex were discontinued a minimum of 4 weeks before random assignment to a double-blind maintenance monotherapy condition.
At each visit, the same psychiatric evaluations administered at the screening visit were administered, and patients were assessed for adverse events. Beginning at week 12 of the open-label acute stabilization phase, patients who met the criteria for entry into the next treatment phase for a minimum of 4 consecutive weeks were eligible to be randomly assigned to a double-blind maintenance monotherapy condition. Entry criteria were a 24-item Hamilton depression scale score ≤20, Young Mania Rating Scale score ≤12, GAS score ≥51, lithium levels ≥0.8 meq/liter, and valproate levels ≥50 μg/ml. Patients not meeting these criteria after 20 weeks were discontinued from the study.
Patients who did not achieve a score of 20 on the 24-item Hamilton depression scale over 4 consecutive weeks during weeks 12–24 while receiving the combination of lithium and divalproex were classified as having refractory depression. Patients who did not achieve a score of 12 or less on the Young Mania Rating Scale over 4 consecutive weeks during weeks 12–24 while receiving the combination of lithium plus divalproex were classified as having refractory hypomania/mania/mixed state.
Patients who missed a total of two visits during the open-label phase met criteria for lack of adherence and were discontinued from the study. Patients not meeting criteria for entry into the maintenance phase and those meeting refractory criteria were discontinued from the study, given six gratis clinical visits over a 3-month period, and were offered either follow-up care within another research study at the investigating site or routine clinical care.
Double-Blind Maintenance Monotherapy Phase
At the beginning of the maintenance phase, patients were assigned 1:1 to treatment with lithium or divalproex monotherapy after stratification for illness type (bipolar I versus bipolar II disorder). Double-blind, double-substitution methodology was used to transition patients from open-label combination therapy with both medications to double-blind monotherapy. Patients were started on equal numbers of capsules of double-blind active lithium 300-mg capsules and matching (in color, taste, and size) lithium placebo capsules, and equal numbers of double-blind active divalproex in 250-mg capsules and matching divalproex placebo capsules.
Patients randomly assigned to monotherapy had one blinded active capsule replaced with a matching placebo capsule once every 2 weeks for as long as necessary. The process of tapering to monotherapy took place over an average of 6 weeks if patients were taking 1200 mg of lithium or 1500 mg of divalproex—longer if the doses of either were higher and more quickly if the doses of either were lower. After the taper was completed, matching placebo for the drug that was discontinued was discontinued for the rest of the maintenance phase. This slow, gradual process of transitioning patients to monotherapy obscured the progress of the taper until completed. The maintenance phase began at the beginning of the taper, and the survival analysis began at that time as well.
After the taper was completed, the number of capsules of active compound and placebo was unchanged for the rest of the maintenance phase, except for adjustments made to both by the unblinded medical monitor when blood levels decreased to less than 0.8 meq/liter for lithium and 50 μg/ml for valproate. Dosing of the active compound could be decreased if patients were believed to be experiencing dose-related side effects (such as tremors) as long as minimum blood levels were maintained. If this was not possible, patients reached study endpoint due to intolerable side effects.
Trough divalproex and lithium levels were performed bimonthly during the first 3 months of the maintenance phase and monthly thereafter. Dose adjustments were made according to blood levels. To maintain the blind and the exact number of capsules being administered during the maintenance phase, each change in the dose of the active compound was accompanied by a matching change in the placebo dose. The number of placebo capsules was decreased commensurately if the number of capsules of the active compound was increased, and vice versa for decreases.
Patients were seen by the research psychiatrist every 2 weeks during the first 3 months of the maintenance phase and monthly thereafter for up to 20 months. At each visit, psychiatric evaluations from the screening visit were repeated, and adverse events were assessed. For patients who experienced no mood episodes for a minimum of 6 months, monthly assessments were continued, but they were then allowed to have assessments conducted over the telephone every other month. Patients could receive lorazepam in doses up to 4 mg/day for anxiety, agitation, and insomnia; as an alternative, adjunctive alprazolam in doses up to 2 mg/day was permitted to treat lorazepam nonresponders. Initiation of psychotherapy was not permitted during the maintenance phase, but patients were permitted to continue any ongoing psychotherapy that had begun before study entry.
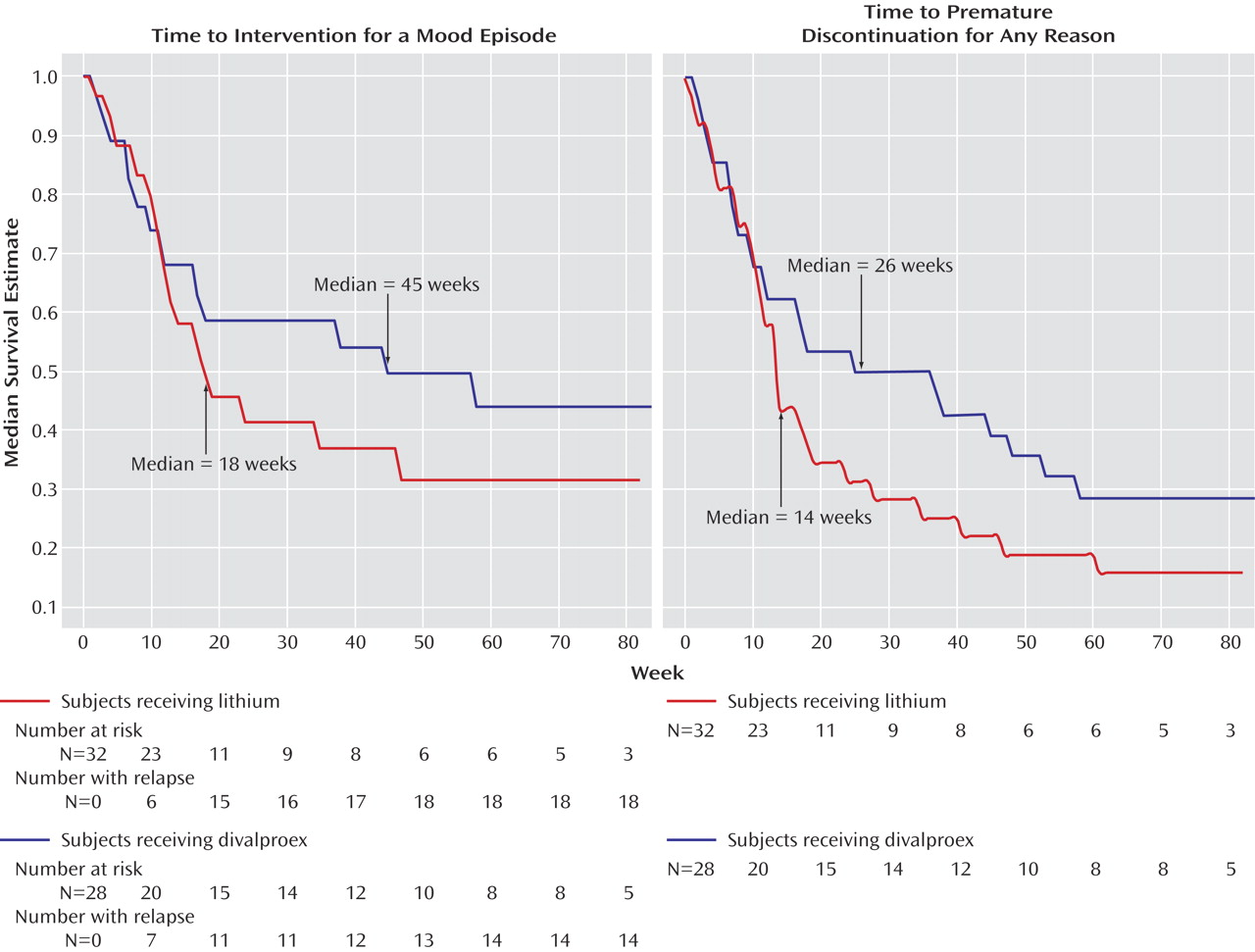
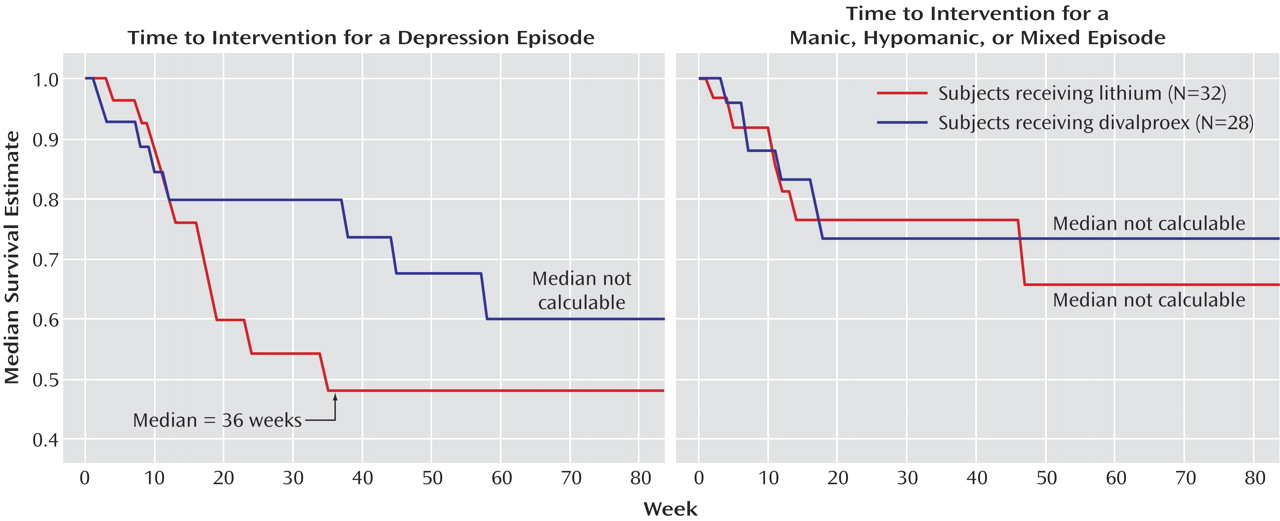
Time to treatment for a mood episode, i.e., time to treatment for emerging symptoms of a relapse at the discretion of the investigators or time to a full relapse was the primary outcome measure for the study. Patients who met criteria for mania (a total Young Mania Rating Scale score ≥20 for up to 8 weeks) or depression (a 24-item Hamilton depression scale score ≥20 for 8 weeks) were considered to have relapsed.
If patients opted for follow-up by the investigators after premature study discontinuation or study completion, they were offered six gratis visits over a 3-month period and then offered routine clinical care or follow-up within another research study at the investigating site. Double-blind study medications were abruptly discontinued and replaced with half the doses of lithium and divalproex prescribed at the end of the open-label acute stabilization phase and then increased to full dosing as tolerated. If patients preferred treatment with the monotherapy provided during the maintenance phase, study medications were continued until an appointment was scheduled with a community psychiatrist who was informed by the unblinded medical monitor of the identity of the medication prescribed during the maintenance phase. Study medications were then abruptly discontinued by the community psychiatrist at the time of this appointment and replaced with the open-label medication the patient was randomly assigned to during the blinded phase of the study.
Data Analysis
The intent-to-treat population included all patients who were randomly assigned to a study treatment condition. Secondary outcome analyses for the maintenance phase were performed on data from all patients who received at least one dose of study drug and had at least one postbaseline outcome assessment during the maintenance phase. The outcome measures included time to additional pharmacotherapy for emerging mood symptoms or full relapse, time to study discontinuation for any reason, time to relapse into depression, and time to relapse into a hypomanic/manic/mixed episode. Kaplan-Meier methodology was used to plot the survival data, and median survival times were calculated. A log-rank test at an alpha=0.05 level of significance was employed to evaluate differences between survival curves. A Cox regression was performed evaluating the following predictors of outcome: treatment arm assignment, type of bipolar diagnosis (bipolar I or bipolar II), and index episode at study entry.
Prior to study initiation, it was estimated that a minimum of 30 patients per arm would be required to detect a minimum hazard ratio of at least 0.36 at a power of 0.80 and an alpha level of 0.05.
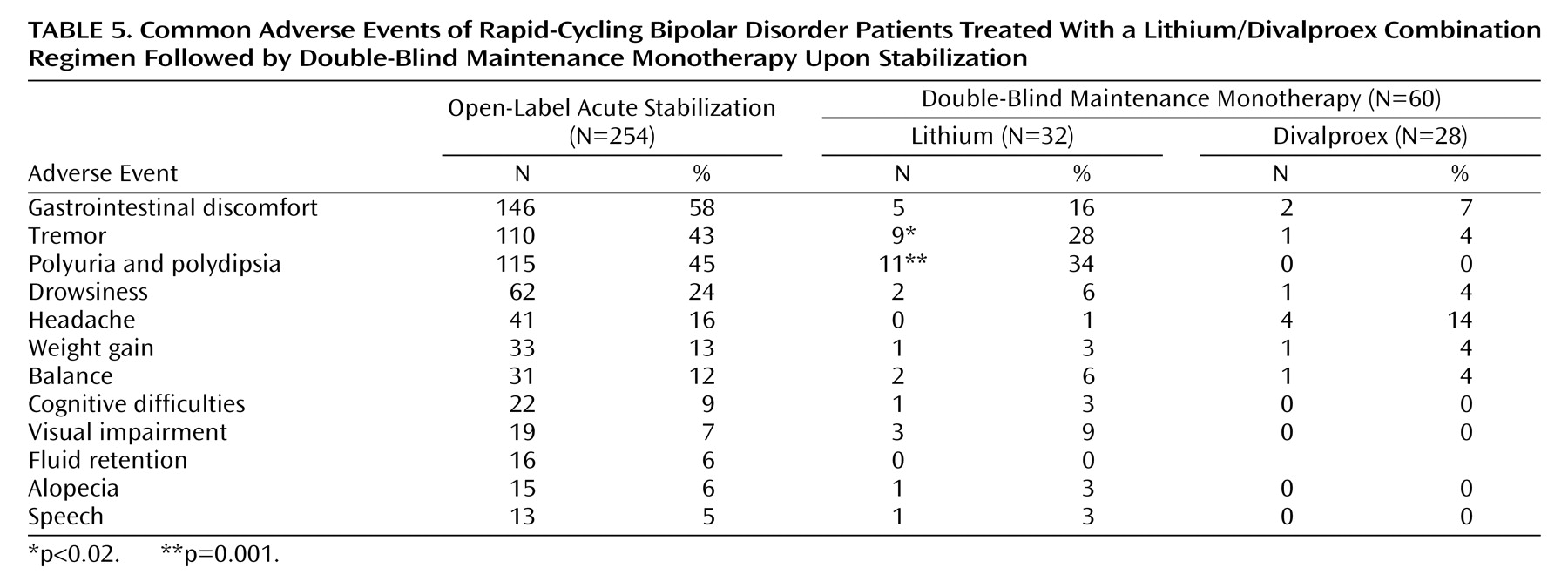
For each study phase, the safety population comprised all patients who received at least one dose of study drug. Safety was assessed by summarizing treatment-emergent adverse experiences and determining changes from the screening visit in clinical laboratory test results, including white blood cell count, platelet count, free thyroxine index, thyroid stimulation hormone, and liver functions tests (ALT and AST).
Discussion
This maintenance monotherapy comparison of lithium and divalproex joins our previous double-blind, long-term evaluation of a population of prospectively defined patients with rapid-cycling bipolar disorder
(11) and is the longest double-blind study conducted in this subgroup of patients.
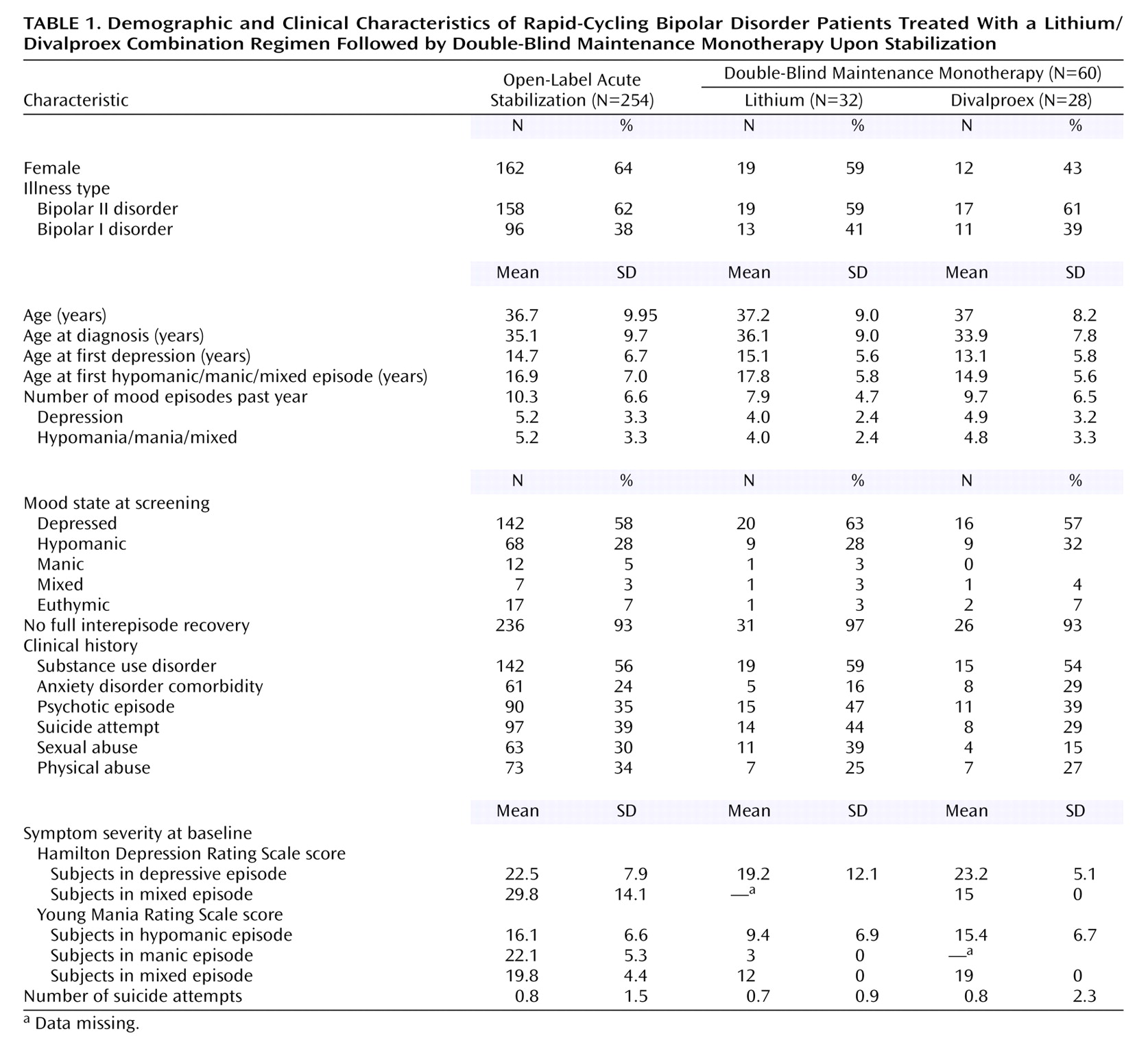
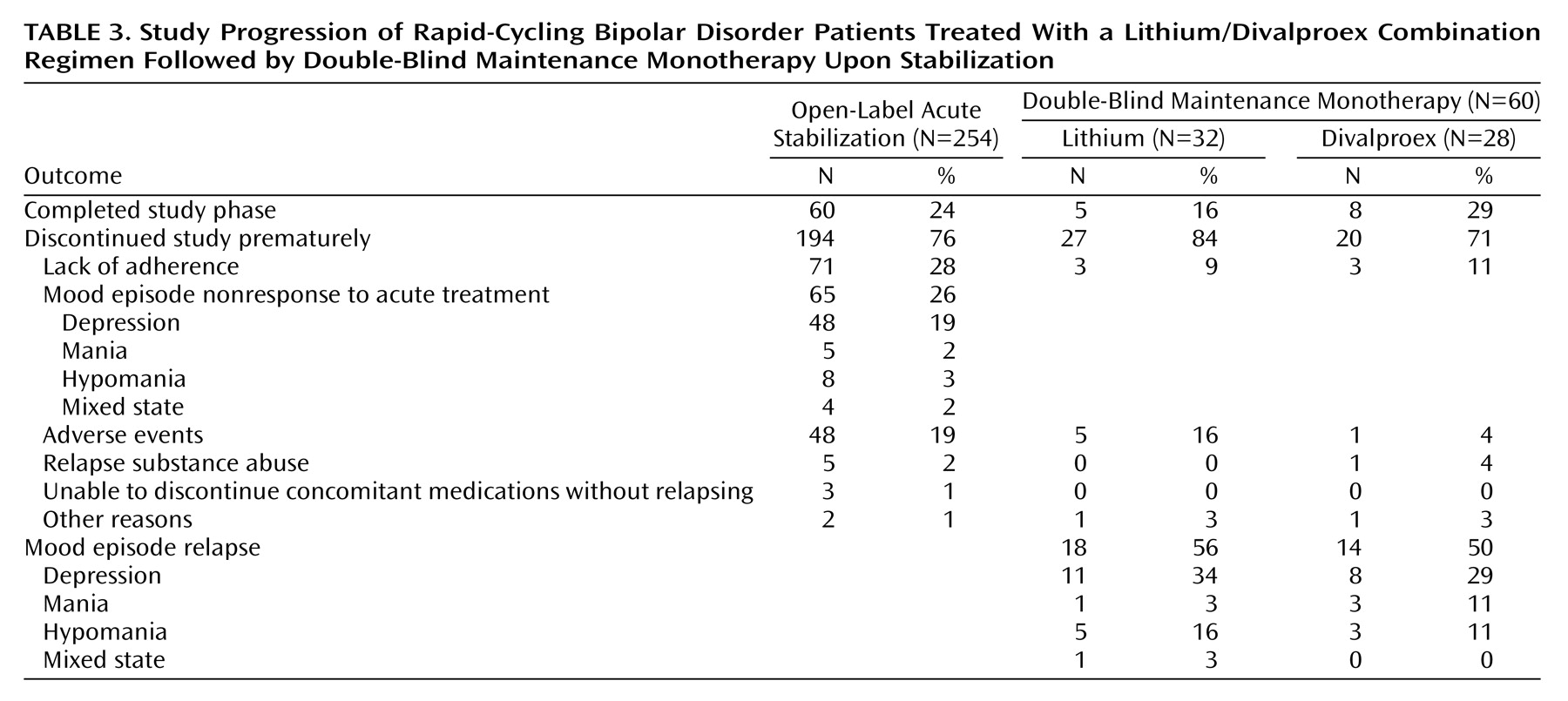
The 254 patients enrolled in this study experienced very severe illness. Only 24% met the rigorously defined response criteria necessary to enter the double-blind maintenance monotherapy phase, which required 4 consecutive weeks of improvement. Of the 65 patients that were not responsive to the treatment combination of lithium plus divalproex, 74% exhibited refractory depression, which suggests that depression while receiving lithium plus divalproex treatment may be a common presentation of patients with rapid cycling.
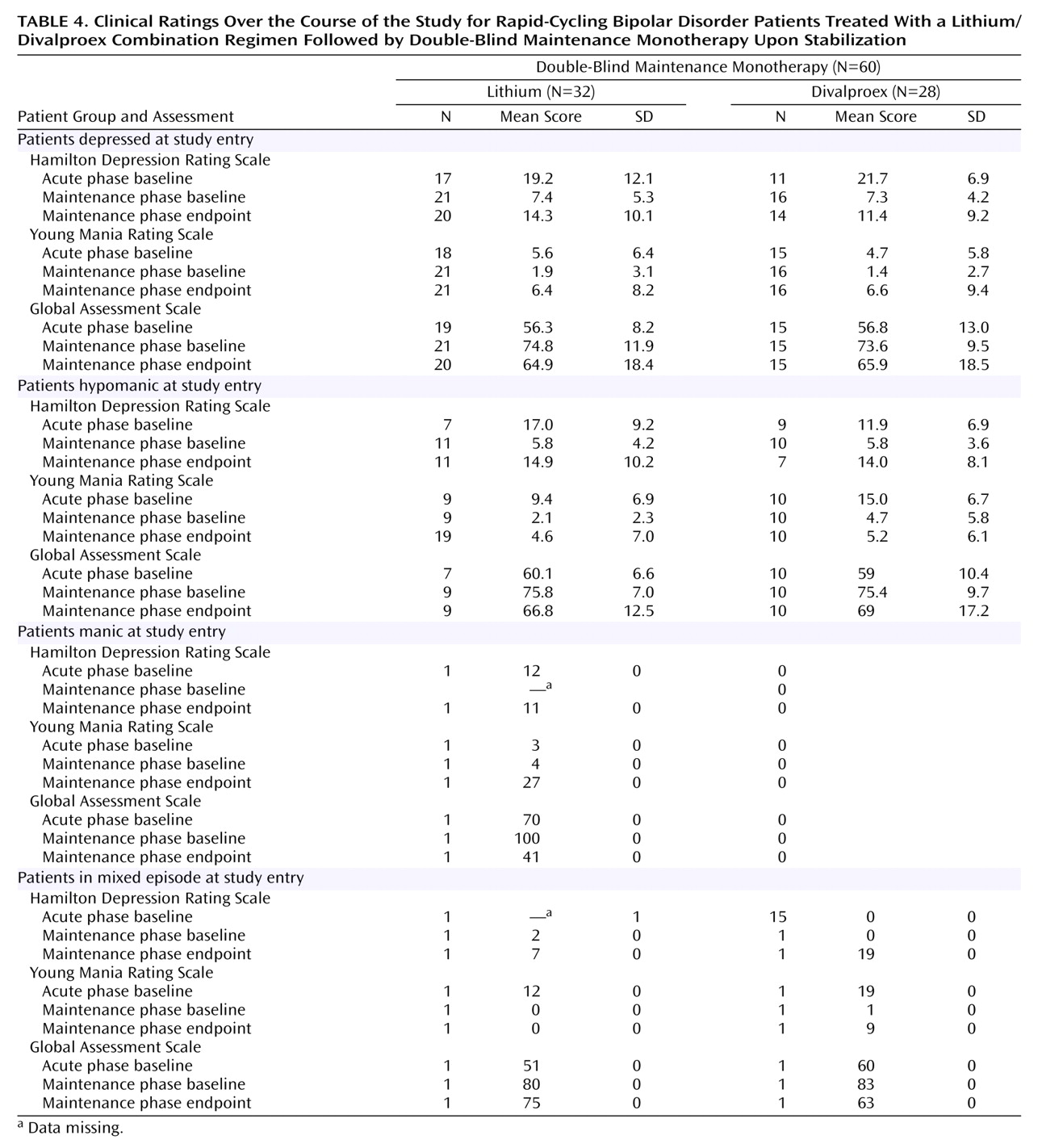
After random assignment, there were no significant differences in rates of relapse into mood episodes or premature discontinuations. The observed differences in survival favoring divalproex over lithium in time to treatment never reached statistical significance. Despite a lifetime of significant morbidity, depressive and manic symptom severity at the time of study entry was only mild to moderate, and there were no significant differences in the worsening of symptom severity and function. Divalproex was significantly better tolerated than lithium as reflected by lower rates of tremors and polyuria/polydipsia, but premature discontinuations due to adverse events did not differ between treatment arms.
These findings do not support the a priori hypothesis that divalproex monotherapy is significantly better than lithium monotherapy for the treatment of rapid-cycling bipolar disorder. Further, the use of the combination of lithium plus divalproex during the open-label acute stabilization phase was only effective in 24% of the intent-to-treat sample, suggesting that three medications or a different combination of two medications may be necessary in the majority of patients with a recent history of rapid cycling.
Several aspects of the design of this study were innovative. First, the open-label acute stabilization phase of this study extended up to 6 months, which is longer than any of the previously conducted maintenance studies in bipolar disorder
(11–
15). Valuable information was obtained on over 250 patients regarding the magnitude and the spectrum of response to the combination of lithium plus divalproex. Second, patients were stabilized with the combination of divalproex and lithium, which are two commonly used treatments for bipolar disorder. As a result, the findings of this study are likely to be meaningful and generalizable. In addition, exposing patients to both agents being compared during the experimental phase of a maintenance study improves generalizability and diminishes bias inherent in comparisons of safety and tolerability, since all subjects had been previously shown to be tolerant of both study medications. Third, entry into the double-blind maintenance monotherapy phase of the study required evidence of improvement over 4 consecutive weeks. While this criterion increased the design’s ability to uncover new mood episodes, it is likely to have been a contributing factor in the study’s low rate of initial phase completion. The criteria to enter the maintenance phase were also rigorous in that they not only concurrently assessed depressive and manic symptom severity but also function. In order to be assigned to a double-blind maintenance monotherapy condition, patients were required to have few or no symptoms of hypomania, but mild symptoms of depression and moderate functional impairment were permitted because of prior data suggesting that the combination of lithium and divalproex would be more effective in managing the symptoms of mania than symptoms of depression
(6). Fourth, the duration of the maintenance phase of this study was 20 months, which is longer than any of the recently conducted maintenance studies in bipolar disorder
(11–
15). As a result, the design assessed rates of relapse over clinically meaningful periods of time.
The design of this study had several methodological limitations. First, the patient group size employed in this study was modest, and as a result it is possible that divalproex may be associated with slightly better prevention of relapse into a syndromal mood episode. The estimated hazard ratio was 0.74, indicating that patients randomly assigned to divalproex had a tendency toward lower risk of relapse (95% CI=0.36 to 1.49). If this estimate were an accurate description of the advantage in preventing a mood episode, then a study would need 364 patients per arm in order to achieve statistical power of 0.80 with alpha set at 0.05, two-tailed. Divalproex showed a tendency toward a larger advantage when both side effects and mood symptoms were considered, with a hazard ratio of 0.68 (95% CI=0.38 to 1.21). If this estimate were accurate, then a study would need to enroll 234 patients per arm to achieve power of 0.80. Second, the design required the unblinded medical monitor to keep lithium levels at a minimum of 0.8 meq/liter and valproate levels at a minimum of 50 μg/ml. This may have disadvantaged the divalproex arm, since recent data suggest that there is a linear relationship between valproate levels and response for acute mania, with the range starting at 71 μg/ml and extending to at least 94 μg/ml
(16). Third, soon after the initiation of this trial it became apparent that the combination of lithium plus divalproex possessed better acute and continuation efficacy for episodes of mania/hypomania than depression. As a result, patients with depressive episodes not responsive to the combination were excluded from the maintenance phase, which limits generalizability. Fourth, although maintenance monotherapy comparisons are a necessary first step in treatment trials, combination therapy designs are needed.
The results from this trial are consistent with rapid cycling being a nonspecific predictor of poor outcome to treatment. The hypothesis that divalproex monotherapy is more effective than lithium monotherapy in the long-term management of rapid-cycling bipolar disorder is not supported by the findings from this maintenance study. These findings suggest that there exists a need for maintenance study designs that combine mood stabilizers possessing a complementary spectrum of activity, including at least one agent that stabilizes mood from below baseline
(17).
The data from this study suggest that refractory depression is probably the most common presentation of rapid-cycling bipolar disorder, even when treated with the combination of lithium plus divalproex. Data from the open-label acute stabilization phase of this study suggest that combination therapy with lithium and divalproex results in marked antimanic but modest antidepressant efficacy. Since the use of antidepressants has been discouraged in the recent treatment guidelines
(18), an alternative study design for future consideration would include the use of a medication such as lamotrigine. Lamotrigine has been shown to stabilize mood, both short-term
(19) and long-term
(13,
14), particularly in patients exhibiting depression with a recent history of rapid cycling
(11). Such a design could compare the efficacy of lithium plus lamotrigine versus divalproex plus lamotrigine. Another possibility would be to consider a design that evaluated the safety and efficacy of triple regimens, i.e., the concurrent use of lithium, divalproex, and lamotrigine versus lithium, divalproex, and a conventional antidepressant.