Negative symptoms are a source of important morbidity in schizophrenia; therefore, developing effective treatments for them is a matter of substantial concern
(1). Since akinesia has been described as a clinical feature common to negative symptoms, retarded depression, and parkinsonism
(2), and since dopaminergic hypofunction has been proposed as a mechanism underlying negative symptoms in schizophrenia
(3,
4), the question arises whether adjunctive use of the dopaminergic antiparkinsonian drug selegiline, a selective monoamine oxidase inhibitor B (MAOI
B), at low doses would be helpful for treating negative symptoms in schizophrenia. Indeed, several open trials have appeared to demonstrate efficacy
(4–
6); however, two small placebo-controlled trials have not
(7,
8). In this araticle we present the results of a large, multicenter, randomized, placebo-controlled study of this question.
Method
A randomized, double-blind trial was carried out at three centers, after approval by each institutional review board: McLean Hospital in Belmont, Mass.; Hillside Hospital in Glen Oaks, N.Y.; and Creedmoor Hospital in Queens, N.Y. Subjects were outpatients meeting DSM-III-R criteria for schizophrenia who understood the nature and purposes of the study as well as its risks, benefits, and alternatives, and who gave their written informed consent to participate. Inclusion criteria included a Scale for the Assessment of Negative Symptoms (SANS) total summary score ≥12, with at least two global subscale scores ≥3; antipsychotic medication treatment ≥1 year, at the current dose ≥1 month, with any other psychotropic medications at a constant dose for ≥1 month. Exclusion criteria included a score ≥5 on any Brief Psychiatric Rating Scale (BPRS) thinking disturbance item; treatment within 1 month of screening with antidepressant medication; and a current diagnosis of major mood or substance abuse disorder. Subjects remained at fixed doses of all psychotropic medications throughout the trial, with the exception of as-needed benzodiazepines, which could be used at clinician discretion.
Subjects underwent a 2-week single-blind placebo run-in, followed by 1:1 random assignment to 12 weeks of treatment with oral selegiline, 5 mg b.i.d., or matched placebo. Subjects were assessed at the end of weeks 1 and 2 of placebo run-in and then every 2 weeks for 12 weeks of active treatment. At each visit subjects were rated with the SANS and BPRS to assess positive symptom severity, Hamilton Depression Rating Scale, Simpson-Angus Rating Scale to assess pseudo-parkinsonian symptom severity, and Clinical Global Impression (CGI) severity and improvement scales to assess global extent of illness and of improvement.
All subjects having at least one postbaseline assessment were included in the analysis. We used random effects regression modeling methods in analyses involving repeated measures within subjects, while controlling for baseline levels of each clinical measure and visit sequence (time). Study-group-by-time interaction was the primary outcome. Statistical significance required two-tailed p<0.05. Data were analyzed by using commercially available statistical packages (Stata, Stata Corp., College Station, Tex., and SAS, SAS Institute, Cary, N.C.).
Results
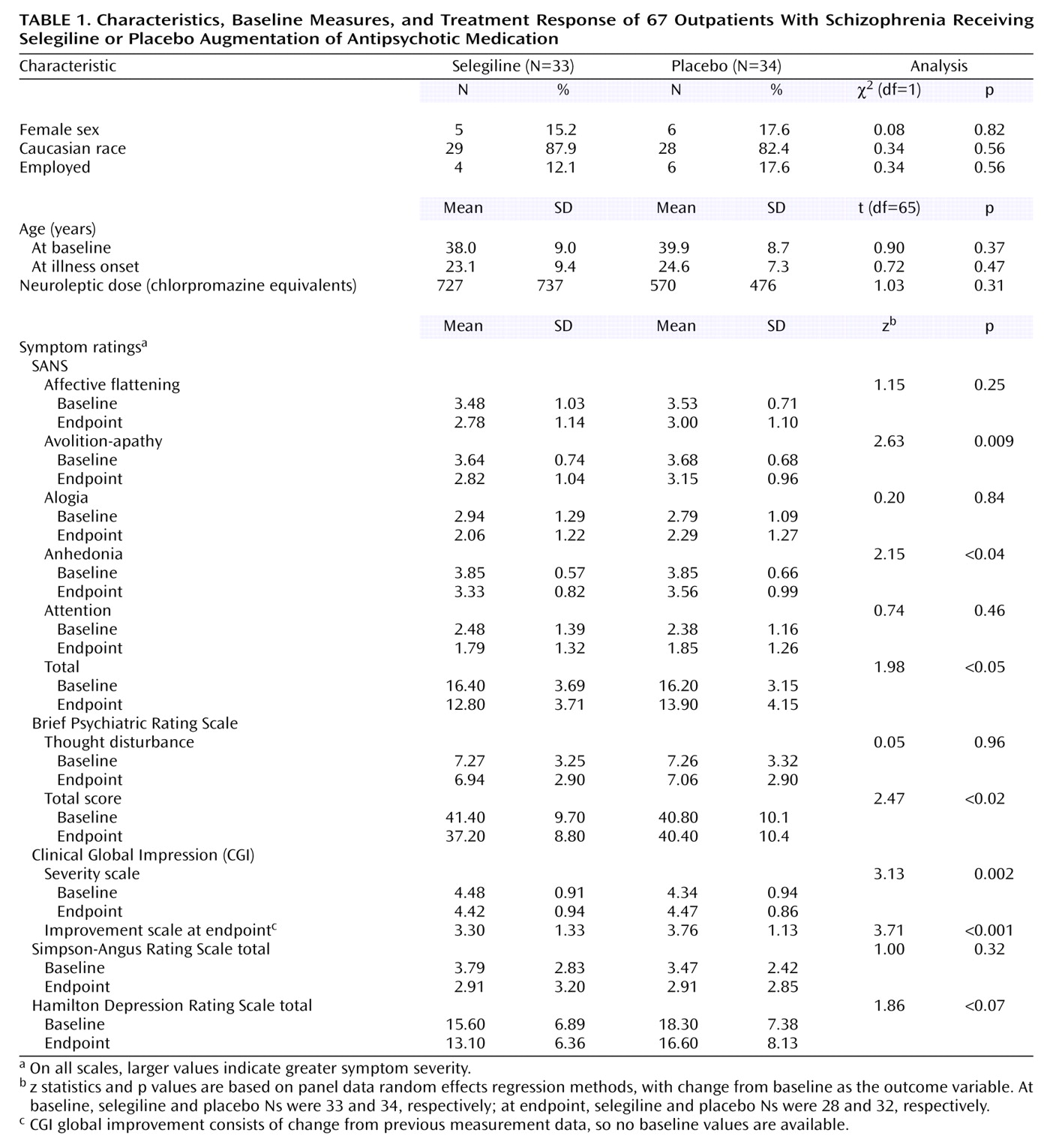
Subjects’ baseline characteristics and treatment responses are summarized in
Table 1. Of 67 subjects randomly assigned to receive placebo or active drug, 33 received selegiline. Treatment groups were well matched demographically for age, race, gender, and employment status as well as age at onset and duration of antipsychotic drug treatment. Baseline clinical characteristics were well matched for all rating scale scores except for the Hamilton depression scale, which showed nonsignificantly greater depression in the placebo group. Neither mean baseline SANS summary total scores nor BPRS thinking disturbance factor scores differed between the active drug and placebo group. Antipsychotic drug dose did not differ significantly between treatment groups, but did among sites (F=4.35, df=1, 64, p=0.04).
The mean duration of study treatment was 11.31 weeks (SD=2.17), and 60 subjects (90%) completed the 12-week trial. Changes favoring selegiline over placebo were found on SANS summary total, avolition-apathy global, and anhedonia global scores; BPRS total score; and CGI severity and improvement scale scores (
Table 1).
No differences emerged between groups on the Simpson-Angus Rating Scale or the BPRS thinking disturbance factor. Treatment effects did not differ by site or gender in random effects regression models.
Because many changes in measures were correlated, it was difficult to calculate an appropriate correction for multiple comparisons and the results are presented without correction. Therefore, the reader should recognize that some findings may represent chance effects.
Discussion
We found improvement in negative symptoms in outpatients with schizophrenia receiving fixed doses of antipsychotic medication when treated with selegiline compared with placebo. There was nonsignificant improvement in depressive symptom severity associated with selegiline treatment and no differential change in extrapyramidal symptoms, suggesting improvement was not attributable to the study drug’s antiparkinsonian effects, though perhaps partly to antidepressant effects
(9). A between-group difference of 0.46 points (SE estimate=0.12) on the CGI improvement scale (
Table 1) indicates that the benefit was clinically meaningful.
MAOIs have been studied in the treatment of apathetic features of schizophrenia since the early 1950s
(4,
10Ð12). There were several findings of beneficial activation but also of overstimulation and psychotic exacerbation, which along with dietary restrictions discouraged the use of these agents in schizophrenia. However, at an MAOI
B-selective dose, which is too low to have consistent antidepressant activity
(9), selegiline augmentation seems to be well tolerated in schizophrenia, as demonstrated in this study by the absence of any worsening of BPRS thought disturbance and the very high rate of completion observed in a 12-week trial.
It should be noted that patients in this study were receiving a wide range of antipsychotic agents and doses, which added “noise” to the results and complicates their interpretation. However, this methodologic feature may also enhance the generalizability of these findings to conditions of actual clinical practice.
In summary, these positive findings support continuing study of selegiline augmentation of antipsychotic medication in patients suffering from chronic schizophrenia with negative symptoms.