Olfactory deficits are recognized as a subtle but common element of schizophrenia pathology
(1) . Behavioral studies of olfaction have focused primarily on impairments of odor identification, with less attention being paid to deficits in other olfactory domains, such as detection threshold sensitivity, discrimination, or memory. Although odor identification deficits appear to be sensitive and specific indicators of both the vulnerability to
(2 –
4) and progressive course of
(5) schizophrenia illness, they are relatively uninformative regarding underlying pathophysiological mechanisms. The ability to correctly identify odors requires more than just an intact olfactory system. It requires a previously learned inventory of recognized odors, the ability to retrieve this inventory, and the ability to associate a retrieved odor memory with a linguistic label. This is a complex, multifaceted, higher-order cognitive operation, and an abnormality at any level of processing can disrupt task performance.
Studies of olfactory acuity or detection threshold sensitivity (i.e., ability to detect a weak odor concentration) focus more directly on olfactory sensory processing isolated from other cognitive aspects of odor identification. Acuity deficits are thought to reflect impairments in the peripheral, as opposed to central, olfactory system. Lesions of the orbitofrontal cortex
(6,
7) or the dorsomedial nucleus of the thalamus
(8), for example, may produce odor identification deficits but leave olfactory acuity intact. Schizophrenia patients exhibit impairments in olfactory acuity, although there have been fewer replications and greater cross-laboratory variability than has been seen for odor identification
(1) .
One limitation of studies of olfactory acuity is that they almost exclusively assess odor detection threshold sensitivity using the single odorant phenyl ethyl alcohol, which is the de facto standard. This is important because odor-specific hyposmias are well documented and are thought to reflect genetic and/or physiological mechanism vulnerabilities associated with specific odor perception
(9) . While detection threshold sensitivity decrements in schizophrenia have typically been interpreted as denoting a generalized acuity deficit, possibly reflecting structural abnormalities in the peripheral olfactory system (e.g., reference
10 ), there is little direct evidence to support this hypothesis. Only two schizophrenia studies have employed another traditional odorant,
n -butanol, rather than phenyl ethyl alcohol. One of these observed a patient deficit
(11), while the other did not
(12) . Similarly, only two studies have examined odor acuity using more than one odorant in the same sample. Both of these reported a differential deficit—i.e., schizophrenia patients had impaired odor detection thresholds to one, but not the other, of the two odorants used in the study. In one case, the odorants differed in the dimension of pleasantness, and patients had reduced sensitivity to the unpleasant odor
(13) . In the other, patients had impaired ability to detect a chemical linked to the unpleasant body odor associated with schizophrenia
(12), an abnormality possibly related to olfactory habituation.
These two studies provide the first evidence to suggest that schizophrenia patients may not, in fact, have a generalized hyposmia that is independent of the specific odorant being tested. However, the subjective dimensions along which the odorants varied—pleasant/unpleasant and body odor/nonbody odor—are not especially informative concerning the neuropathological mechanisms that might underlie these odor-specific deficits. In the current study, we examined odor acuity in schizophrenia patients using odorants that differed in a more precise physiological manner—the ability to increase intracellular cAMP. Typically, when an odorant binds to a receptor on the membrane of an olfactory receptor neuron in the nasal epithelium, it induces g-protein mediated activation of adenylyl cyclase, increasing intracellular cAMP. This, in turn, causes cyclic nucleotide-gated ion channels to open, resulting in neuronal depolarization. There is a strong positive correlation (r=0.89), across a wide range of odorants, between odor-stimulated adenylyl cyclase activity and the summed electrical response of the olfactory epithelium (electro-olfactogram or EOG). This association confirms the essential role of cAMP in mediating olfactory signal transduction
(14) . However, the magnitudes of these two correlated responses are highly variable across odorants. While some elicit robust activity, others elicit only minimal responses
(14,
15) . Of importance, there is also a strong positive correlation between these in vitro adenylyl cyclase and EOG voltage responses and the subjective perception of odor intensity in vivo. When healthy subjects rated the intensities of different odorants, all presented at the same suprathreshold concentration, their subjective ratings correlated significantly with both adenylyl cyclase activity and EOG amplitude
(16) . These in vivo, in vitro associations establish the validity of these odor-specific in vitro responses for translational human studies of olfactory processing.
We selected this dimension of adenylyl cyclase activation as our “dimension-of-interest” because a growing body of evidence suggests that intracellular cAMP signaling pathways may be dysregulated in schizophrenia
(17 –
20) . Perhaps more compelling is the fact that DISC1, the schizophrenia susceptibility gene on chromosome 1q42, sequesters phosphodiesterase 4B (PDE4B), the enzyme that inactivates cAMP, in an inactive state and releases it when cAMP levels increase
(19) . Although this evidence remains indirect, it clearly supports the hypothesis that dysregulated cAMP signaling may contribute to schizophrenia pathology
(21) . We theorized that if this is indeed the case, then it might be observable in differential acuity deficits for odorants that differentially activated the intracellular cAMP signaling cascade. We further hypothesized that given the genetic associations that appear to underlie cAMP dysregulation
(19 –
21), similar differential acuity deficits might be observed in unaffected first-degree relatives of schizophrenia probands.
Method
Subjects
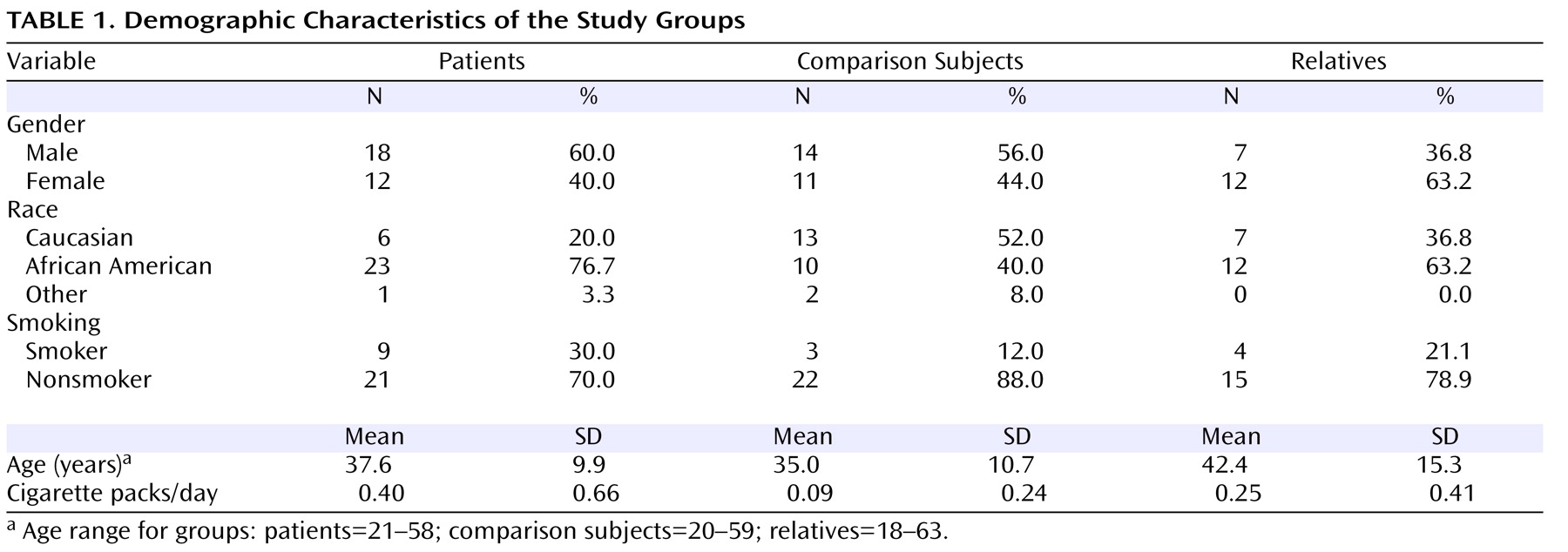
The sample included 30 patients with DSM-IV-diagnosed schizophrenia, 25 healthy comparison subjects, and 19 unaffected first-degree relatives of patients. All healthy comparison subjects were unrelated individuals. Unaffected family members included five parents, 13 siblings, and one adult child of a schizophrenia patient, from 17 independent families. Two siblings came from one family, as did one sibling and one parent. The patient sample included five affected probands linked to seven of these relatives, plus 25 unrelated individuals. All patients were stable outpatients at the time of testing.
All subjects received the Diagnostic Interview for Genetic Studies and the Family Interview for Genetic Studies. Patients were excluded for any concurrent axis I diagnosis other than schizophrenia. Healthy comparison subjects were excluded for any history of an axis I diagnosis, axis II cluster A personality disorder, or family history of axis I psychotic disorder in a first-degree relative. Family members were excluded for any axis I psychotic disorder or prodromal psychotic symptoms but not for a previous nonpsychotic axis I disorder if it resolved more than 1 year ago and was not associated with any current pharmacotherapy. Nor were they excluded for an axis II cluster A diagnosis. Among the 19 family members, two had histories of a prior depressive episode; none met criteria for schizoid or schizotypal personality disorder. Subjects were excluded for any history of neurological disorder, head trauma with loss of consciousness, lifetime history of substance dependence, substance abuse within the preceding 6 months, or any medical condition that might affect cerebral functioning. Subjects were also excluded for any obvious cranio-facial trauma or abnormality, including septal deviation, and any acute respiratory condition, cold, or allergy. Written informed consent was obtained after all procedures were fully explained, in compliance with guidelines established by the University of Pennsylvania Institutional Review Board.
Demographic characteristics of the groups are presented in
Table 1 . There were no significant differences in sex distribution (χ
2 =12.67, df=2, p=0.26) or mean age (F=2.18, df=2, 71, p=0.12). There were also no overall differences in smoking status, whether assessed in terms of the number of active smokers within each group (χ
2 =2.61, df=2, p=0.27) or the mean number of packs smoked per day (F=2.75, df=2, 71, p=0.07). In paired contrasts, however, patients smoked significantly more than healthy comparison subjects (F=4.97, df=1, 53, p=0.03), while family members did not differ from either group.
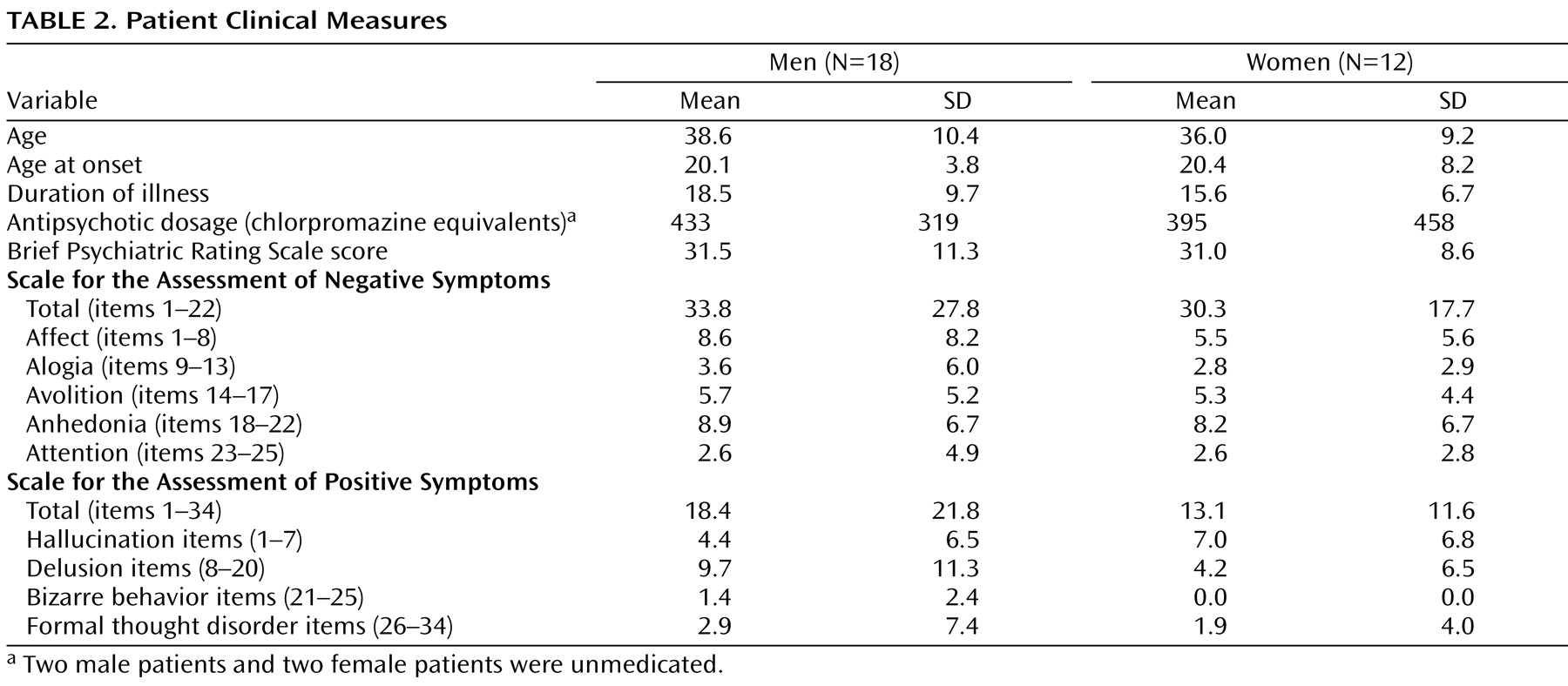
Descriptive clinical information and standardized rating scale measures for patients are presented in
Table 2 . The Brief Psychiatric Rating Scale (BPRS)
(22), the Scale for the Assessment of Negative Symptoms
(23), and the Scale for the Assessment of Positive Symptoms
(24) were obtained at the time of testing. Ratings were completed by trained investigators with an interrater reliability of >0.90. BPRS items were summed to form an index of overall symptom severity. Scale for the Assessment of Negative Symptom global ratings for five negative symptom subscales (affective flattening, alogia, anhedonia, avolition, attention) and Scale for the Assessment of Positive Symptoms global ratings for four positive symptom subscales (hallucinations, delusions, bizarre behavior, formal thought disorder) assessed specific dimensions of psychotic symptom profiles. Overall, these ratings suggested a mild level of both positive and negative symptoms. Twenty-six of 30 patients were taking antipsychotic medications. Medication dosages were calibrated across subjects as chlorpromazine equivalents
(25) .
Selection of Odorants
The two odorants employed in the study were citralva (3,7-dimethyl-2,6-octadienenitrile) and lyral (4-[4-hydroxy-4-methylpentyl]-3-cyclohexene-1-carboxyaldehyde). These are both volatile organic compounds used as fragrance additives in many commercial products. Citralva has a molecular weight of 149.2 and a density of 0.859–0.870 g/ml. Lyral has a molecular weight of 210.3 and a density of 0.985–0.993 g/ml. They are qualitatively similar in having pleasant floral/fruity aromas. However, they differ markedly in the extent to which they activate the intracellular signaling cascade mediating chemosensory signal transduction in olfactory receptor neurons. The response elicited by citralva is quite strong, while the response elicited by lyral is relatively weak. In a study of odor-stimulated adenylyl cyclase activity, using ex vivo chemosensory cilia preparations, citralva ranked fourth in the magnitude of its adenylyl cyclase response, while lyral ranked 42nd out of 44 odorants
(15) . Similarly, in a study of the odor-stimulated EOG response, citralva ranked fifth out of 36 odorants, whereas lyral ranked 21st in the magnitude of this electrical response
(14) .
Experimental Procedures
Standardized psychophysical assessments of the subjects’ ability to detect citralva and lyral were conducted after they refrained from smoking for approximately 1 hour. Separate tests of right and left nostril sensitivity were conducted for each odorant, while the other nostril was occluded with durapore tape (3M Corporation, Minneapolis, Minn.)
(26) . For a given subject, testing began with a specific odor-nostril combination followed by presentation of the same odorant to the opposite nostril. The second odorant was then presented to each nostril in the same order as the first. Test order was counterbalanced across subjects for both initial odorant and nostril.
A single staircase, forced-choice task was used to estimate detection threshold sensitivity. In this task, the subjects were asked to smell two vials, one containing pure mineral oil and the other containing an active odorant diluted in mineral oil, and identify which vial “smells stronger.” Concentrations of both lyral and citralva ranged from 10 –1 M (strongest) to 10 –9 M (weakest), in 0.5 log step dilution increments. The test began at the 10 –5 M step, and odor concentration was increased in full-molar steps until correct detection (i.e., active odorant identified as stronger) occurred on five consecutive trials at a given concentration. Odor concentration was then increased or decreased in half-molar increments, depending upon performance on two trials at each concentration step (i.e., odor concentration was decreased after 2 correct trials and increased after an incorrect trial). The geometric mean of the last four staircase reversal points (out of seven) was taken as the estimate of odor detection threshold sensitivity (i.e., the weakest odor concentration that could be reliably identified as stronger than mineral oil). The average number of odorant exposures to achieve a stable measure of detection threshold sensitivity was 12.6 (SD=2.9); this did not vary across diagnostic groups, odorants, or nostrils. Total test time was approximately 30 minutes.
Statistical Analysis
Since the patient and family member groups were not strictly independent samples, group differences were assessed using the generalized linear latent and mixed models (GLLAMM) algorithm implemented in Stata 9.0 (Statacorp, College Station, Tex.), with subject and family as hierarchically nested random-effects factors. This effectively accounted for any shared variance between individual members of the same family. Group (patient/healthy comparison subject/relative), gender, nostril (left/right), odorant (citralva/lyral), and age were fixed-effects predictors. Significance levels of individual model parameters were assessed using the Wald test statistic with chi-square distribution. Significant group differences and interactions were parsed by post hoc computation of appropriate linear combinations of model coefficients, along with associated z-statistics. Post hoc contrasts were subject to Bonferroni-corrected p values of p<0.05 to minimize type I errors.
Results
The initial analysis revealed a significant effect of group (χ
2 =7.31, df=2, p=0.02), odor (χ
2 =44.85, df=1, p<0.0001), and group-by-odor interaction (χ
2 =12.22, df=2, p=0.002). There were no significant effects of sex (χ
2 =0.90, df=1, p=0.34), age (χ
2 =1.95, df=1, p=0.16), or nostril (χ
2 =0.04, df=1, p=0.85). When smoking status was included as a moderating factor, either as an indicator variable (smoker/nonsmoker) or as a continuous measure (packs/day), it was not significantly related to odor acuity (indicator: χ
2 =0.38, df=1, p=0.54; continuous: χ
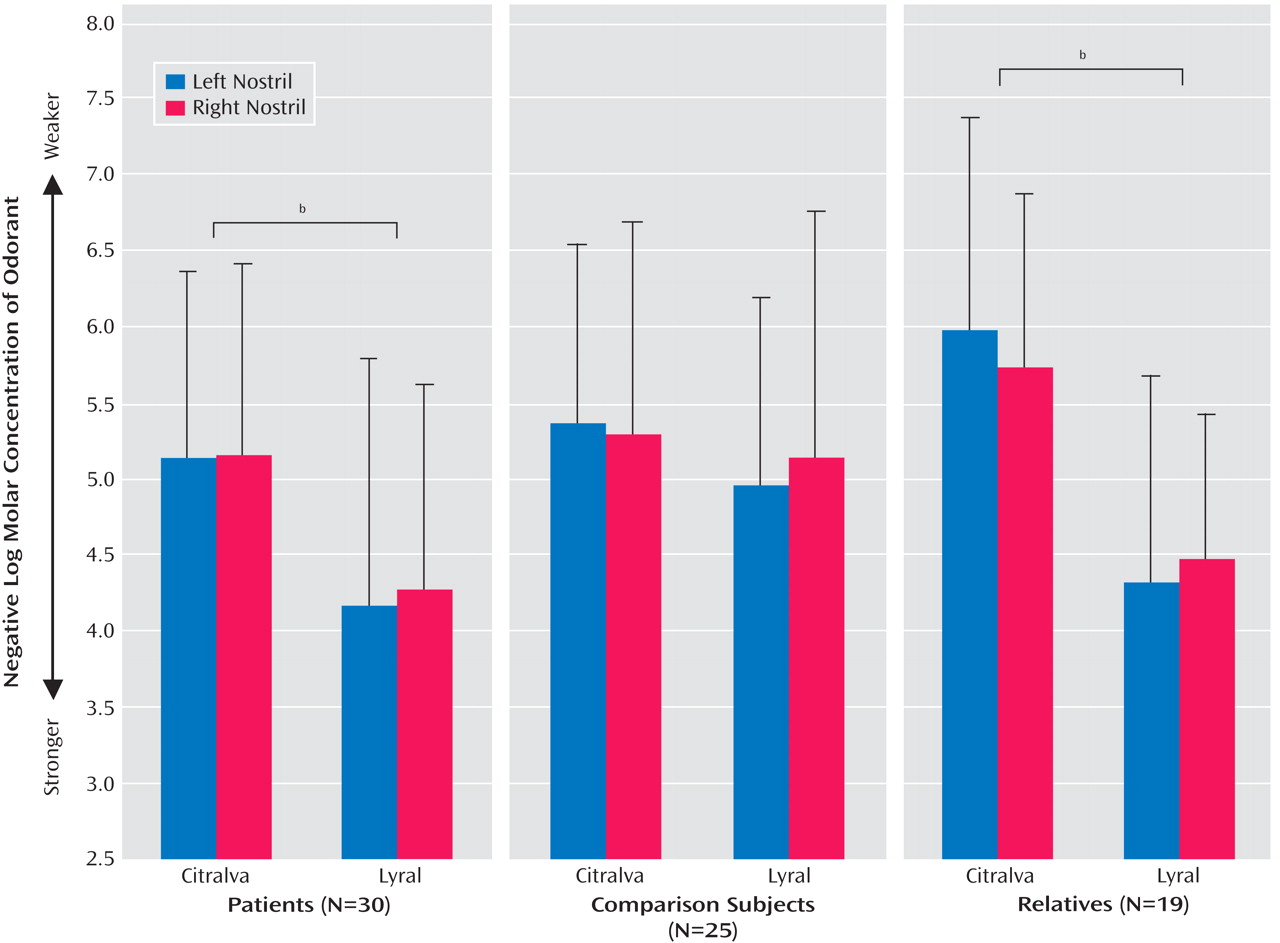
2 =0.79, df=1, p=0.37), and it did not alter any of the other significant effects. Mean odor detection threshold sensitivities for each group, odor, and nostril are presented in
Figure 1 .
To determine if schizophrenia patients exhibited differential acuities to citralva and lyral as a result of the odors’ differential effects on cAMP signaling, we parsed the group-by-odor interaction by contrasting threshold detection sensitivities to the two odors separately within each group. Both schizophrenia patients (χ
2 =20.31, df=1, p<0.001) and unaffected family members (χ
2 =31.91, df=1, p<0.001) had differential acuity impairments to lyral relative to citralva. Healthy comparison subjects showed no difference in their relative sensitivity to the two odorants (χ
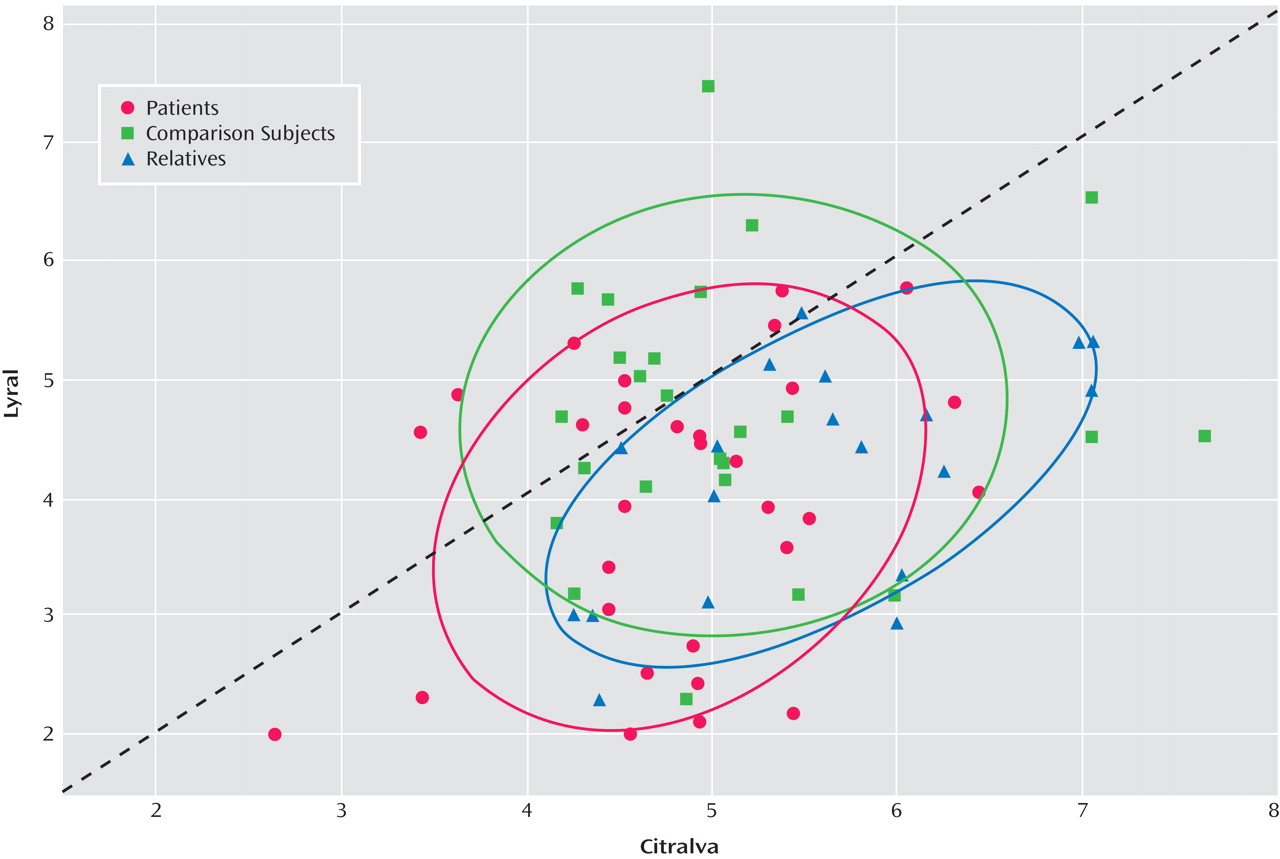
2 =1.54, df=1, p=0.64). Effect sizes (Cohen’s d) for this citralva-lyral difference were 0.86 for patients and 1.47 for family members, indicating a large effect for both groups. The relatively larger effect size within the family group reflected their greater sensitivity to citralva but a comparable impairment in their ability to detect lyral. As illustrated in
Figure 2, a small number of patients were markedly impaired in their ability to detect citralva as well as lyral.
We considered whether there were relationships, among patients, between odor acuity measures and measures of clinical symptom profiles or treatment status. Odor threshold sensitivities were dependent measures in a composite general linear model (GLM) with the following independent measures: Scale for the Assessment of Negative Symptoms and Scale for the Assessment of Positive Symptoms subscale ratings, antipsychotic medication dosage, age of illness onset, duration of illness, and total BPRS score. None of these clinical measures were related to any of the olfactory measures, even with an uncorrected value of p<0.05 for an exploratory analysis.
Discussion
This study clearly demonstrates that schizophrenia patients and their unaffected first-degree relatives exhibit odor-specific acuity deficits for lyral but not citralva. These cannot be explained by nonspecific factors such as age, sex, smoking history, acute symptom profile, or antipsychotic medication. Given that these two odorants are distinguished by their abilities to activate the cAMP intracellular signaling cascade-mediating olfactory neuron depolarization, this odor-specific hyposmia may indicate a genetically regulated disruption of cAMP-mediated signal transduction. As noted in the introduction, there is growing evidence to suggest that cAMP is dysregulated in schizophrenia
(18 –
20) . While not dispositive of such a mechanistic disturbance, the results of this study are clearly consistent with this hypothesis. Of importance, this hypothesis is not inconsistent with other models of schizophrenia pathophysiology. Altered cAMP levels can be secondary to either glutamatergic
(27) or dopaminergic
(28) dysregulation.
There are, however, alternative explanations that must be considered. The original studies demonstrating differential cAMP responses to citralva versus lyral
(14,
15) examined aggregate multicellular responses. A more recent study of single cell responses confirmed that citralva induces a transmembrane current that is approximately twice the magnitude of that induced by lyral
(29) . This study also indicated, though, that fewer olfactory neurons respond to lyral than to citralva. It is possible, therefore, that reduced sensitivity to lyral simply reflects the fact that fewer neurons are being stimulated. However, there are several reasons to think that this is not the case. First, if threshold detection sensitivity reflects the total number of neurons capable of responding to a given odorant, then we should see a lyral-citralva acuity difference in healthy subjects, as well as in schizophrenia patients and family members. These individuals, though, had identical thresholds for the two odorants. Conversely, if schizophrenia patients have a simple loss of olfactory receptor neurons that impairs their odor detection sensitivity, then they should exhibit impairments to both lyral and citralva. There is no reason to expect selective loss of neurons that respond to lyral but not citralva, since receptor-specific neurons are distributed diffusely throughout the olfactory epithelium and any given odorant is capable of binding to several different receptors. Finally, the observation that more neurons respond to citralva than to lyral was made using suprathreshold concentrations of odorants. By definition, odor detection threshold represents the odor concentration required to stimulate the minimum number of receptors to enable an odor to be detected. It is likely that this minimum required number of activated neurons is relatively the same across odorants and is not dependent upon the total number of neurons capable of responding to higher odor concentrations. The total number of response-capable neurons is more likely to affect the perceived intensity of a suprathreshold odorant, which was found to be greater for citralva even in healthy subjects
(16) .
There is also evidence to suggest that intracellular pathways other than cAMP may contribute to mammalian olfactory signal transduction. The presence of alternate signaling pathways has been well-established in nonmammalian species (e.g., reference
30 ) and also proposed for human olfaction
(31) . Only recently, though, has it been clearly demonstrated that cAMP-independent pathways are capable of mediating odor perception in the mammalian olfactory system
(32) . We cannot rule out the possibility that lyral and citralva are mediated through different intracellular signaling mechanisms, with one being impaired and one remaining intact. However, we think this is unlikely for the following reasons. While disruption of cAMP-mediated signaling does not result in complete anosmia, cAMP remains the principal mediator of olfactory receptor neuron responses. Decreased cyclic nucleotide levels in the nasal mucosa are associated with impaired odor threshold sensitivities
(33) . In the absence of an intact cAMP pathway, only a subset of previously detectable odors remains detectable, and the magnitudes of the responses evoked by these odorants are reduced exponentially. Of most importance, there is no detectable response to lyral following disruption of the cAMP pathway
(32) . So, despite establishing the functional importance of alternate signal transduction pathways, these data actually support the hypothesis that impaired ability to detect lyral reflects dysregulation of the cAMP pathway.
If this olfactory deficit does denote a disturbance in cAMP signaling, why would the deficit be expressed only in response to an odorant associated with lower levels of cAMP activity? The explanation is likely related to the fact that fluctuations in basal concentrations of cAMP cause olfactory signal transduction to be intrinsically noisy
(34) . Since the magnitude of the lyral-evoked response is relatively small
(29), it is more difficult to distinguish this response from baseline fluctuations than it is to distinguish the response evoked by citralva. If this odor-evoked response is further attenuated in schizophrenia patients relative to healthy individuals, this would further reduce the signal-to-noise ratio, resulting in an undetectable near-baseline response. The response to citralva might be similarly attenuated but, being intrinsically larger, still remain strong enough to produce reliable signal detection.
We must emphasize, though, that studies implicating cAMP dysregulation in schizophrenia have not clearly established the direction of this functional dysregulation. Although we have discussed it in terms of an attenuated response, the data remain inconsistent. For example, given the ability of DISC1 to sequester PDE4B in an inactive state, a genetic abnormality in DISC1 should decrease PDE4B sequestration which, in turn, would decrease cAMP. However, a genetic abnormality in PDE4B itself, which disrupts PDE4B-mediated inactivation of cAMP and results in increased cAMP levels, has also been associated with schizophrenia
(18) . The complexity of the situation is illustrated by the animal model in which mice expressing a constitutively active isoform of Gas exhibit prepulse inhibition deficits. As predicted from its ability to stimulate adenylyl cyclase, this active isoform increased cAMP levels in the striatum. However, because of compensatory increases in PDE4B, cAMP levels in the forebrain were decreased
(20) . Whether cAMP levels are increased or decreased in the olfactory system remains unknown, although the results of this study are most consistent with a functional decrement.
These findings clearly need to be replicated using other odorants associated with high versus low adenylyl cyclase activity to confirm that this is, in fact, the “dimension-of-interest” underlying this differential deficit. Additional physiological, rather than behavioral, studies are also needed. We would anticipate, based on these behavioral findings, that the EOG depolarization response recorded from the nasal epithelium would be similarly disrupted following stimulation with lyral but not citralva. Studies using olfactory epithelial biopsy material obtained from schizophrenia patients could also be employed to clarify the relationships between behavioral deficits, electrophysiological responses, and biochemical pathways in the olfactory receptor neurons. Nevertheless, the results of this study offer strong inferential support for the hypothesis that cAMP signaling is dysregulated in schizophrenia.