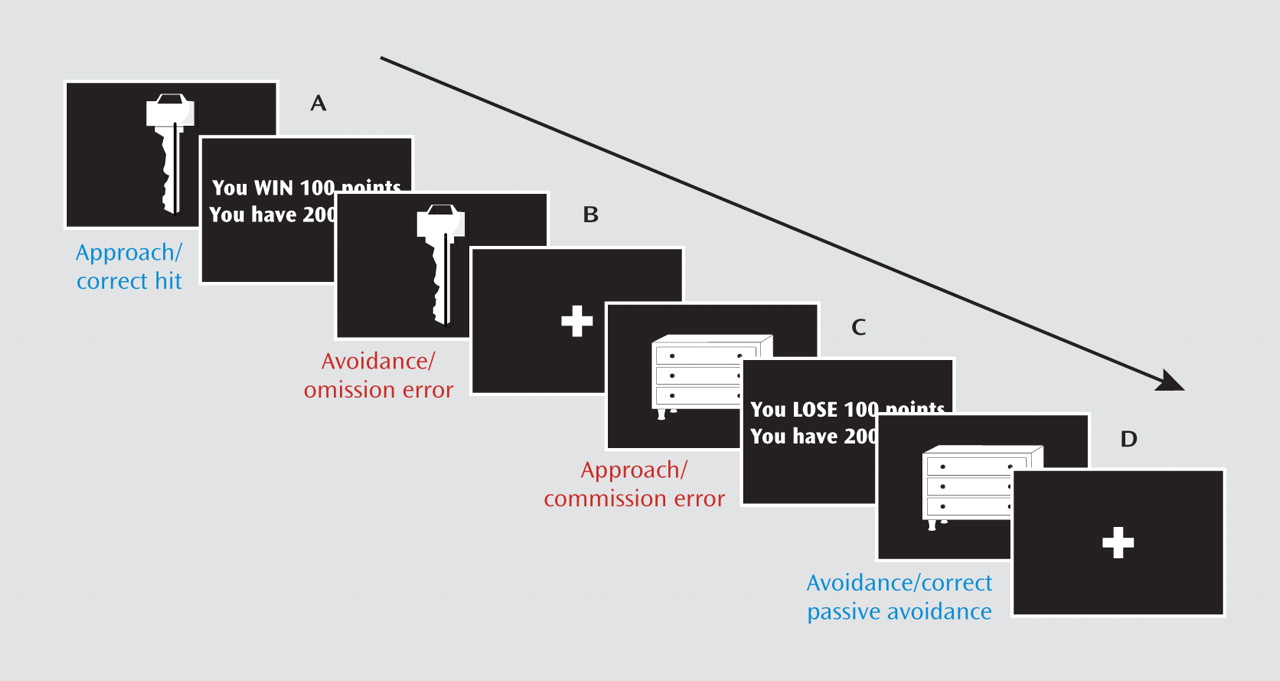
Passive avoidance learning tasks are particularly informative, as both electrophysiologic and lesion studies map its neural basis, implicating the amygdala, orbitofrontal cortex, and striatum (
17–21). In addition, a functional magnetic resonance imaging (fMRI) study of a passive avoidance task has confirmed the importance of these regions in healthy human adults (
22). It is argued that the amygdala allows the formation of stimulus-reinforcement associations, determining which of the stimuli are “good” and which “bad,” and that this information is fed forward as reinforcement expectations to the orbitofrontal cortex (
19–21), potentially via the ventral striatum (
23). The orbitofrontal cortex is critical for the representation of this reinforcement-expectation information, thereby facilitating successful decision making (
24,
25). The orbitofrontal cortex and caudate are also critical for error prediction, which facilitates learning of reward or punishment contingencies (
26–28). The detection of prediction errors prompts enhanced reinforcement-based learning (
29). These prediction errors are maximal after the individual's first exposure to the cue and reinforcement but rapidly lessen (unless the reinforcement contingency changes) as the individual develops an expectation of the reinforcement associated with the cue (
29).
Originally, the deficits shown by individuals with psychopathic traits on passive avoidance tasks were attributed to less processing of punishment information (
11). However, work has shown that individuals with psychopathic traits appropriately use punishment information to modify responses immediately following an error. Instead, it is argued that the deficits arise from impairment in the formation and use of stimulus-reinforcement associations in decision making (
30). The individual with psychopathic traits is less able to use reinforcement information to change his or her representation of the outcome associated with the response and consequently is less able to use reinforcement expectancies to guide behavior (
13). A deficit in stimulus-reinforcement processing is thought to result from dysfunction in the integrated functioning of the amygdala and orbitofrontal cortex (
13). In line with this model, recent fMRI work has indicated less amygdala responding and less amygdala-orbitofrontal cortex functional connectivity in response to fearful expressions in youths with psychopathic traits than in comparison subjects (
8,
9). Moreover, anomalous neural responses in the orbitofrontal cortex to reversal errors have also been identified in this population (
10).
The goal of the current study was to assess the nature of the functional irregularities within the amygdala, orbitofrontal cortex, and striatum of youths with conduct disorder or oppositional defiant disorder plus psychopathic traits. Specifically, the study tested three hypotheses. First, we hypothesized that early reinforcement processing would be abnormal in these youths. For initial exposures to reinforced outcomes, prediction errors are high; the participant has no reinforcement expectancy associated with the stimulus, and thus all reinforcement received is unexpected (
29). Healthy individuals show marked increases in activity, particularly within the orbitofrontal cortex and striatum, in response to unexpected reinforcements (
26–28). We predicted, however, that this initial heightened responding to early trials would not be seen in the subjects with conduct disorder or oppositional defiant disorder plus psychopathic traits, at least within the orbitofrontal cortex. Our second hypothesis was that the signaling associated with reward and punishment outcomes would be abnormal in this group. Several studies have documented heightened responses to rewarded relative to punished outcomes within the amygdala, orbitofrontal cortex, and striatum during decision making (
23,
24,
31). We predicted that this heightened responding to rewarded outcomes would be seen only in the healthy youths and not in those with conduct disorder or oppositional defiant disorder plus psychopathic traits.
Discussion
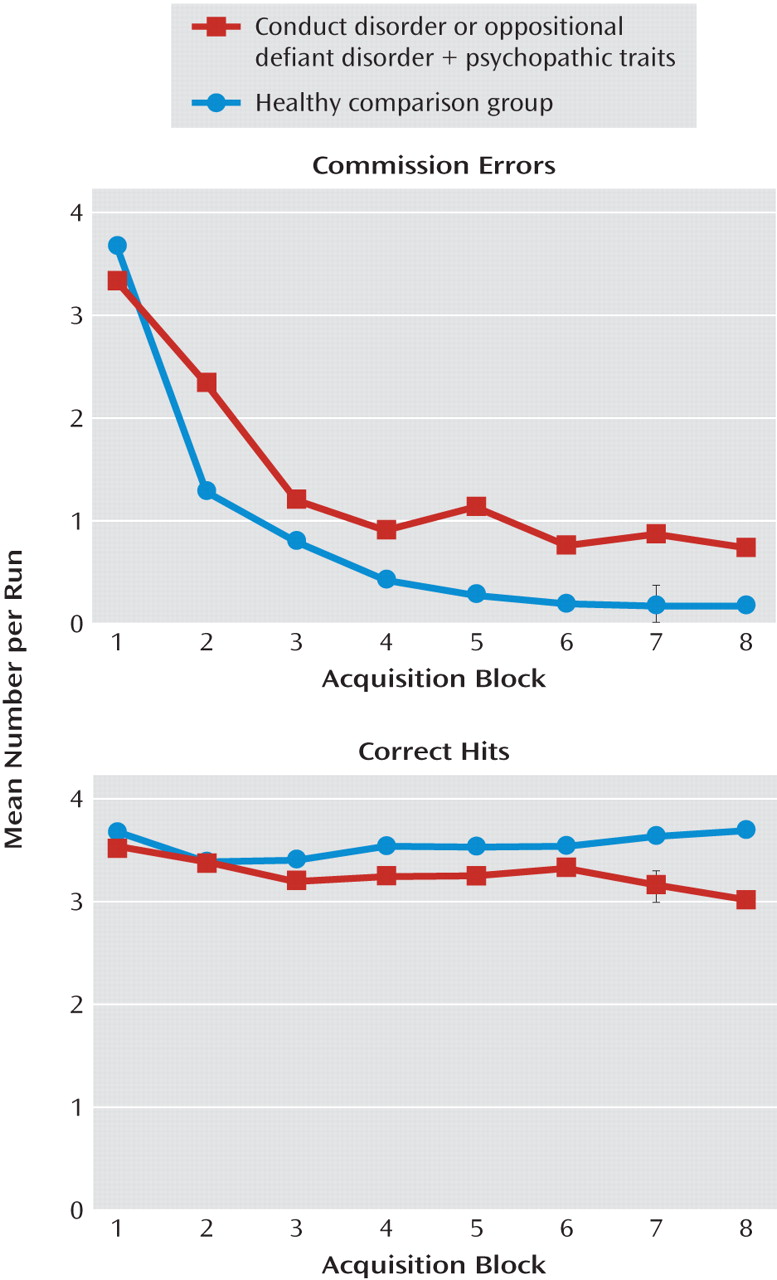
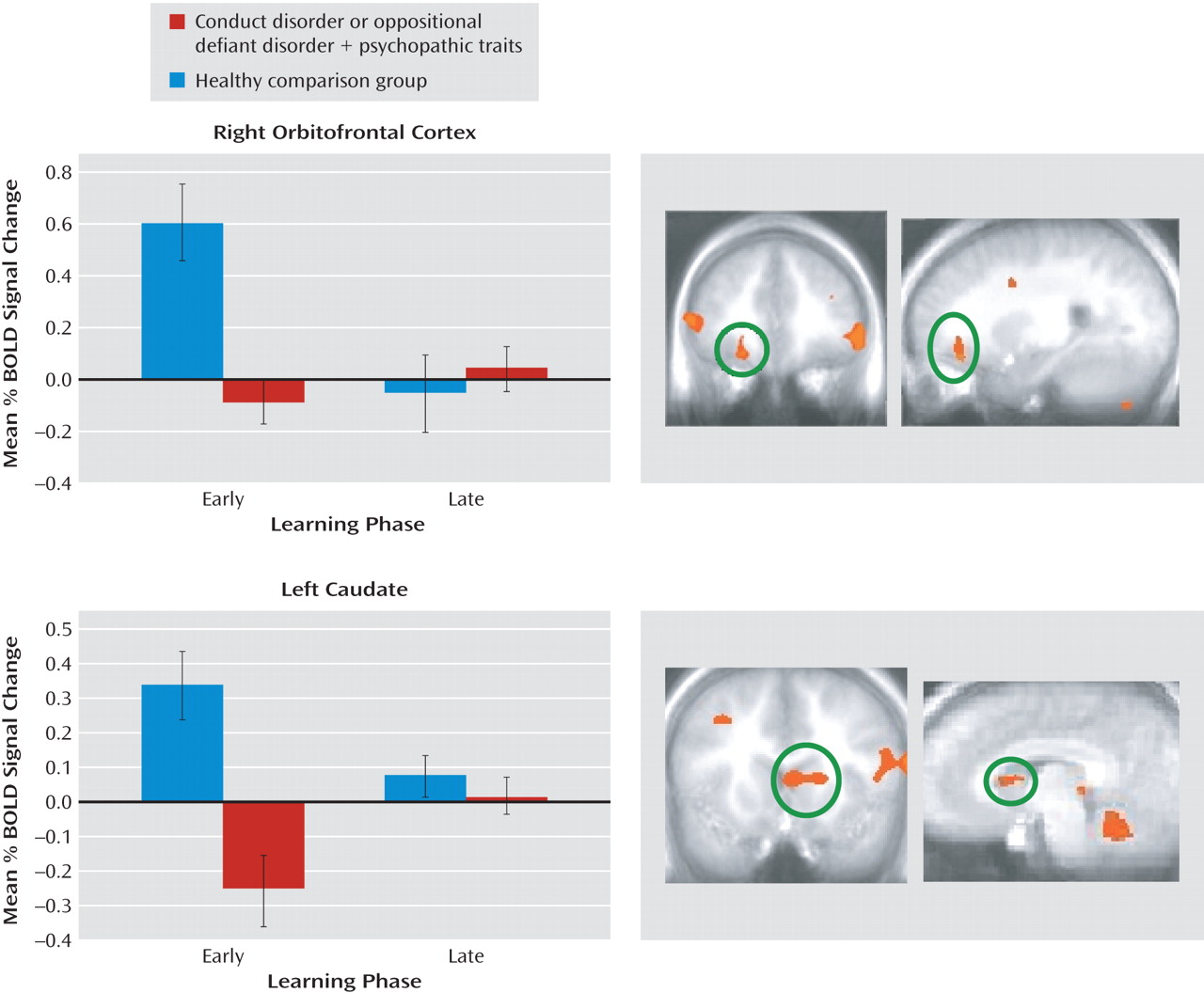
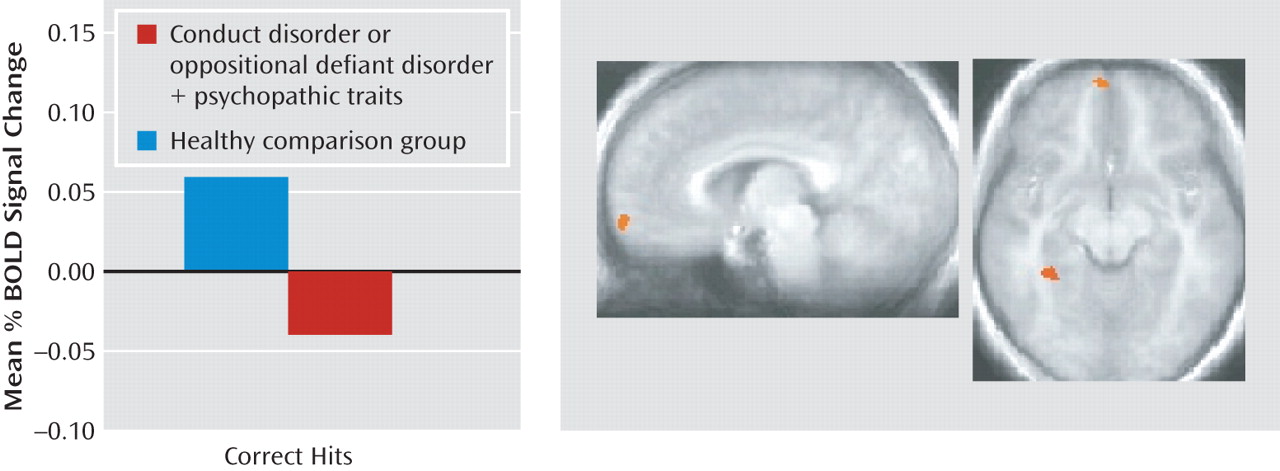
The goal of the current study was to assess the nature of the irregularities within the amygdala, orbitofrontal cortex, and striatum in youths with conduct disorder or oppositional defiant disorder plus psychopathic traits during passive avoidance learning. Specifically, this study examined group differences in neural responses to two features critical to emotional learning: the level of experience with the stimulus (early versus late trials) and the type of response and reinforcement (rewarded correct responses versus punished incorrect responses). The results revealed that the youths with psychopathic traits, relative to healthy youths, showed significantly less activity within the orbitofrontal cortex and caudate in response to early exposures to reinforced outcomes. Moreover, in response to rewarded correct responses, the group with psychopathic traits showed significantly less activity within the orbitofrontal cortex than did the comparison youths. These task measures did not reveal group differences within the amygdala, although the group with psychopathic traits had generally lower activity within this region across the task.
Impairments in passive avoidance have been repeatedly reported in individuals with psychopathic traits (
11,
15,
16,
44). Our model of passive avoidance learning and its dysfunction in individuals with psychopathic traits is highly influenced by the work of Schoenbaum, Gallagher, and colleagues (
19,
45,
46). In essence, we assume that in healthy individuals prediction errors in response to unexpected reinforcements (mediated by the orbitofrontal cortex and caudate) serve to initiate rapid stimulus-reinforcement learning in the amygdala. As learning progresses, more accurate reinforcement expectancies are fed from the amygdala to the orbitofrontal cortex, where their representation allows successful decision making to occur, initiating approaches to objects associated with reward and avoidance of those associated with punishment. In individuals with high levels of psychopathic traits, deficits in prediction error signaling lead to slower and weaker stimulus-reinforcement learning, which in turn leads to weaker and less accurate reinforcement expectancies being fed forward to the orbitofrontal cortex. Individuals become progressively less accurate in their decisions relative to comparison individuals because they are less able to represent the reward expectancy value associated with the presented stimulus and respond accordingly (
13).
Previous work has indicated dysfunction of the orbitofrontal cortex in individuals with psychopathic traits (
47,
48). The current study extends this by providing information on the nature of the functional impairment. In line with the model, youths with conduct disorder or oppositional defiant disorder plus psychopathic traits, relative to comparison youths, showed significantly lower orbitofrontal cortex responses during early trial learning and to rewarded responses. The first of these findings is compatible with the suggestion of disrupted prediction error signaling in the orbitofrontal cortex in the youths with psychopathic traits (see reference
28). Prediction errors are maximal after the individual's first exposure to the relationship between the cue and the reinforcement but rapidly lessen as the individual develops an expectation of the reinforcement associated with the cue (
29). In this regard it is notable that we have previously documented lower orbitofrontal cortex responses, relative to those in comparison groups, to unexpected failures to receive rewards in children with conduct disorder or oppositional defiant disorder plus psychopathic traits (
10). The finding of lower orbitofrontal cortex responses to rewarded responses is consistent with the suggestion that this population is less able to represent reward expectancy values. As such, these data are consistent with results from a previous study examining youths with conduct disorder, undifferentiated by psychopathic traits (
49). This study also showed lower responsiveness to reward outcome information within the orbitofrontal cortex in the youths with conduct disorder.
Neurobiological accounts of psychopathic traits (
13,
50,
51) have neglected consideration of the caudate (and for that matter, the ventral tegmental area and ventral striatum), despite the critical role of these regions in prediction error signaling and/or reinforcement-based learning (
26,
27,
52). Part of this likely reflects the choice of cognitive functions examined in the majority of previous fMRI work with youths or adults with psychopathic traits, i.e., expression processing (
8,
9,
53), emotional memory (
54), and moral reasoning (
55). However, the current study revealed lower responsiveness in the caudate (but not the ventral tegmental area or ventral striatum) to early reinforced outcomes relative to later trials in the youths with psychopathic traits. This is compatible with the suggestion of disrupted prediction error signaling in the caudate in youths with conduct disorder or oppositional defiant disorder plus psychopathic traits. Indeed, it is notable that the only previous study examining operant responding in individuals with psychopathic traits also showed dysfunctional responding to reversal errors in the caudate but not ventral striatum among children with conduct disorder or oppositional defiant disorder plus psychopathic traits (
10). This pattern is echoed by recent structural MRI work also indicating selective structural abnormalities within the dorsal striatum in adults with psychopathic traits (
56). A recent study of healthy adults focused on the nucleus accumbens and found that individuals scoring higher on the impulsive aggressivity components of the Psychopathic Traits Inventory had greater pharmacologically triggered dopamine release and greater activity in response to reward (
57). Although intriguing results, these data were somewhat inconsistent with those from the only previous study of which we are aware that showed ventral striatal abnormalities in adults meeting criteria for psychopathy (
54). This latter study showed
lower ventral striatum activity in response to emotional stimuli in the adults with psychopathy—albeit in response to a very different task. The current study also did not identify any regions demonstrating greater activity during rewarding trials in the youths with psychopathic traits. These differences may reflect the hypothesized distinction between individuals showing heightened aggressive impulsivity in the absence of the core callous traits of psychopathy (who are believed to demonstrate heightened limbic responsiveness [14]) and those who do show these core callous traits (who show low limbic responsiveness).
The dorsal anterior cingulate cortex has been implicated in conflict monitoring (
58) and the use of error signals and the integration of reinforcement history to select between competing responses (
59,
60). The current study was not designed to distinguish between these models. However, it is important to note that the significant main effect of response type, i.e., commission error versus correct hit, identified in this region (see table in online data supplement) was not modulated by a main effect of group or a group-by-response type interaction. These data suggest that the responses of the dorsal anterior cingulate cortex to the conflict initiated by punishment/error feedback remain intact in youths with conduct disorder or oppositional defiant disorder plus psychopathic traits. Notably, this replicates prior results in similar subjects during a reversal learning paradigm, where both groups showed appropriate recruitment of the dorsal anterior cingulate cortex in response to punished reversal errors (
10). In contrast, functional (
10) and now structural (
56) abnormalities have been reported in these populations in the dorsal striatum (caudate), indicating that abnormalities in reward processing may arise from deficits in the amygdala-insula-orbitofrontal-caudate circuit.
The main effect of diagnosis revealed reduced activation in several brain regions in the youths with psychopathic traits. This could indicate less engagement with the task. In contrast, the regions showing a main effect of response type and phase (i.e., regions where both groups showed appropriate responses as a function of task measures) include the dorsal anterior cingulate cortex and inferior frontal cortex/anterior insula. It is important to note that these data suggest that an engagement explanation appears unsatisfactory, as there is no reason why less task engagement would lead to appropriate recruitment of systems necessary for response change but selectively less recruitment of regions implicated in decision making based on stimulus reinforcement.
One caveat of the present study is that although there were high rates of comorbidity of attention deficit hyperactivity disorder (ADHD) with conduct disorder and oppositional defiant disorder, we did not include a second ADHD comparison group in the current study because neither of our previous studies indicated that youths with ADHD have pathophysiology in the amygdala or orbitofrontal cortex, although this was seen in children with conduct disorder or oppositional defiant disorder plus psychopathic traits (
8,
10). Rubia and colleagues have shown dysfunction in orbitofrontal cortex reward signaling in youths with conduct disorder who do not have ADHD but not in youths with ADHD (
49). Second, the youths with conduct disorder or oppositional defiant disorder plus psychopathic traits made significantly more commission errors during the late learning phase, which could produce stronger parameter estimates for late commission errors for this group. However, it should be noted that the groups differed not in BOLD responses to late commission errors but, rather, in responses during early trials and in responses to rewarded correct hits.
In short, the current results are highly compatible with developmental models of conduct disorder with psychopathic traits that stress that this disorder reflects a fundamental disruption in the neural systems necessary for appropriate decision making in regard to stimulus reinforcement (
14). Problems in this basic form of emotional learning will create difficulties for the child in socializing; the child will be less likely to “learn from his or her mistakes.” Moreover, children will learn from emotional feedback more slowly—this is notable in the present study both in the behavioral data and in the lower sensitivity within both the orbitofrontal cortex and the caudate to early reinforcement information. Appropriate representation of such information is critical for rapid, early emotional learning. In addition, the dysfunction in the signaling of reinforcement outcomes in the orbitofrontal cortex, documented in this study, will be associated with poor decision making. This will lead to the selection of nonoptimal behavioral choices (actions that harm others and actions that are likely to result in individuals being frustrated and potentially reactively aggressive because they do not meet their goals).
These data have clear treatment implications. Specifically, they suggest that the development of pharmacologic or behavioral therapeutic approaches that augment the capacity to make decisions based on stimulus reinforcement may enable youths with high levels of psychopathic traits to overcome their functional deficiencies. In this regard, it is notable that both serotonergic (
61) and dopaminergic (
62) manipulations influence the performance of the task described in this study as well as its neural substrates. Moreover, a recent report indicated that prediction error signaling in the amygdala facilitates learning by increasing orienting and attention to the stimulus during the subsequent trial (
63), and an interesting behavioral approach might be to train youths to direct their attention to explicitly form predictions about the expected reward from a particular action and to explicitly attend to the difference between their verbalized prediction and the actual outcome for both advantageous and disadvantageous actions.
In summary, the current data support previous suggestions of dysfunction in the amygdala and orbitofrontal cortex in youths with conduct disorder or oppositional defiant disorder plus psychopathic traits. More important, they indicate that such individuals are marked by a compromised sensitivity to early reinforcement information in both the orbitofrontal cortex and caudate and to reward outcome information within the orbitofrontal cortex. In conjunction with established models of stimulus-reinforcement instrumental learning, they suggest that the integrated functioning of the amygdala, caudate, and orbitofrontal cortex may be disrupted in individuals with conduct disorder or oppositional defiant disorder plus psychopathic traits.