Copy Number Variants in Schizophrenia: Confirmation of Five Previous Findings and New Evidence for 3q29 Microdeletions and VIPR2 Duplications
Abstract
Objective:
Method:
Results:
Conclusions:
Method
Subjects and DNA Specimens
| Subjects in Molecular Genetics of Schizophrenia Study (MGS) | Comparison Subjects (Children's Hospital of Philadelphia)b | ||||||
|---|---|---|---|---|---|---|---|
| Group | Total | DNA From Lymphoblastic Cell Lines | DNA From Blood | Subjects in International Schizophrenia Consortium (ISC) Data Set With CNVs >100 kba | Assayed With Illumina 550K Array (13) | Assayed With Illumina 610K Arrayc | All Subjects |
| Subjects with schizophrenia or schizoaffective disorder | |||||||
| European ancestry | 2,671 | 1,998 | 673 | 3,391 | 6,062 | ||
| African American | 1,274 | 1,004 | 270 | 0 | 1,274 | ||
| Total | 3,945 | 3,002 | 943 | 3,391 | 7,336 | ||
| Comparison subjects | |||||||
| European ancestry | 2,648 | 2,623 | 25 | 3,181 | 886 | 3,532 | 10,243 |
| African American | 963 | 962 | 1 | 0 | 582 | 3,029 | 4,574 |
| Total | 3,611 | 3,585 | 26 | 3,181 | 1,464 | 6,561 | 14,817 |
| All subjects | 7,556 | 6,587 | 5319 | 6,572 | 1,464 | 6,561 | 22,153 |
GWAS Assay and Detection of CNVs
CNV Quality Control Analyses
CNV call quality control.
Quality control of DNA samples.
Visual confirmation.
Quantitative polymerase chain reaction.
Analysis of CNV Association
Additional CNV Data
Statistical Analyses and Thresholds of Significance
Clinical Data
Results
| Group or Analysis | VIPR2: Chromosome 7, 158.51-158.63 Mb, Exonic Duplications | AGTPBP1: Chromosome 9, 87.35-87.55 Mb, Exonic Duplications | GLB1L3/2: Chromosome 11, 133.65-133.69 Mb, Exonic Deletions | C16orf72: Chromosome 16, 9.09-9.12 Mb, Exonic Duplications | NEDD4L: Chromosome 18, 53.86-54.22 Mb, Exonic Duplications | 3q29 Multigenic CNV: Chromosome 3, 197.2-198.83 Mb, Deletions | 3q26.1 Intergenic CNV: Chromosome 3, 165.61-165.66 Mb, Deletions | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| With | Without | With | Without | With | Without | With | Without | With | Without | With | Without | With | Without | |
| Molecular Genetics of Schizophrenia study (MGS) (current study) | ||||||||||||||
| European-ancestry case subjects | 7 | 2,664 | 4 | 2,667 | 6 | 2,665 | 8 | 2,663 | 0 | 2,671 | 4 | 2,667 | 2 | 2,669 |
| European-ancestry comparison subjects | 1 | 2,647 | 0 | 2,648 | 2 | 2,646 | 0 | 2,648 | 0 | 2,648 | 0 | 2,648 | 0 | 2,648 |
| African American case subjects | 3 | 1,271 | 1 | 1,273 | 9 | 1,265 | 2 | 1,272 | 6 | 1,268 | 1 | 1,273 | 3 | 1,271 |
| African American comparison subjects | 1 | 962 | 0 | 963 | 1 | 962 | 0 | 963 | 1 | 962 | 0 | 963 | 0 | 963 |
| Total case subjects | 10 | 3,935 | 5 | 3,940 | 15 | 3,930 | 10 | 3,935 | 6 | 3,939 | 5 | 3,940 | 5 | 3,940 |
| Total comparison subjects | 2 | 3,609 | 0 | 3,611 | 3 | 3,608 | 0 | 3,611 | 1 | 3,610 | 0 | 3,611 | 0 | 3,611 |
| p | Odds Ratio (CI) | p | Odds Ratio (CI) | p | Odds Ratio (CI) | p | Odds Ratio (CI) | p | Odds Ratio (CI) | p | Odds Ratio (CI) | p | Odds Ratio (CI) | |
| Meta-analysis, Cochran-Mantel-Haenszel exact testa | 0.03 | 0.04 | 0.009 | 0.002 | 0.12 | 0.04 | 0.05 | |||||||
| One-sided odds ratio (CI, lower bound)b | 4.6 (1.2) | (1.1) | 4.3 (1.4) | (2.7) | 4.5 (0.7) | (1.1) | (1.0) | |||||||
| Two-sided odds ratio (CI) | 4.6 (1.0-42.9) | (0.9) | 4.3 (1.2-23.2) | (2.1) | 4.5 (0.6-209.5) | (0.9) | (0.8) | |||||||
| Pooled analysis, Fisher's exact testa | 0.03 | 0.04 | 0.007 | 0.002 | 0.08 | 0.04 | 0.04 | |||||||
| One-sided odds ratio (CI, lower bound)b | 4.6 (1.2) | (1.1) | 4.6 (1.5) | (2.6) | 5.5 (0.8) | (1.1) | (1.1) | |||||||
| Two-sided odds ratio (CI) | 4.6 (1.0) | (0.8) | 4.6 (1.3-24.8) | (2.1) | 5.5 (0.7-252.7) | (0.9) | (0.9) | |||||||
| With | Without | With | Without | With | Without | With | Without | With | Without | With | Without | With | Without | |
| International Schizophrenia Consortium study (ISC)c | ||||||||||||||
| Case subjects | 4 | 3,387 | — | — | — | — | — | — | — | — | 2 | 3,389 | — | — |
| Comparison subjects | 0 | 3,181 | — | — | — | — | — | — | — | — | 0 | 3,181 | — | — |
| p | Odds Ratio (CI) | p | Odds Ratio (CI) | |||||||||||
| Meta-analysis of MGS and ISC studies, Cochran-Mantel-Haenszel exact testd | 0.004 | 0.01 | ||||||||||||
| One-sided odds ratio (CI, lower bound)b | 6.4 (1.8) | (1.8) | ||||||||||||
| Two-sided odds ratio (CI) | 6.4 (1.5-58.3) | (1.4) | ||||||||||||
| With | Without | With | Without | With | Without | With | Without | With | Without | With | Without | With | Without | |
| Comparison subjects (Children's Hospital of Philadelphia)e | ||||||||||||||
| European ancestry | 3 | 4,415 | 5 | 4,413 | 6 | 4,412 | 1 | 3,531 | 0 | 4,418 | 0 | 4,418 | 1 | 4,417 |
| African Americans | 2 | 3,609 | 1 | 3,610 | 6 | 3,605 | 1 | 3,028 | 4 | 3,607 | 0 | 3,611 | 3 | 3,608 |
| Total subjects from all studies | ||||||||||||||
| Case subjects | 14 | 7,322 | 5 | 3,940 | 15 | 3,930 | 10 | 3,935 | 6 | 3,939 | 7 | 7,329 | 5 | 3,940 |
| Comparison subjects | 7 | 14,814 | 6 | 11,634 | 15 | 11,625 | 2 | 10,170 | 5 | 11,635 | 0 | 14,821 | 4 | 11,636 |
| Proportion of case subjects | 0.0019 | — | 0.0013 | — | 0.0038 | — | 0.0025 | — | 0.0015 | — | 0.0010 | — | 0.0013 | — |
| Proportion of comparison subjects | 0.0005 | — | 0.0005 | — | 0.0013 | — | 0.0002 | — | 0.0004 | — | — | 0.0003 | — | |
| p | Odds Ratio (CI) | p | Odds Ratio (CI) | p | Odds Ratio (CI) | p | Odds Ratio (CI) | p | Odds Ratio (CI) | p | Odds Ratio (CI) | p | Odds Ratio (CI) | |
| Pooled analysis (Fisher's exact test) | 0.002 | 0.12 | 0.003 | 0.0001 | 0.04 | 0.0004 | 0.05 | |||||||
| One-sided odds ratio (CI, lower bound)b | 4.0 (1.8) | 2.5 (0.7) | 3.0 (1.5) | 12.9 (3.3) | 3.5 (1.1) | (3.8) | 3.7 (1.0) | |||||||
| Two-sided odds ratio (CI) | 4.0 (1.5-11.9) | 2.5 (0.6-9.7) | 3.0 (1.3-6.5) | 12.9 (2.8-121.4) | 3.5 (0.9-14.7) | (2.9) | 3.7 (0.8-18.6) | |||||||
| Range | Range | Range | Range | Range | Range | Range | ||||||||
| Size (kb) | 76-648 | 88-117 | 20-91 | 22-900 | 82-93 | 1.6 | 6-66 | |||||||
| Number | Number | Number | Number | Number | Number | Number | ||||||||
| MGS subjects with CNV >100 kb | 11/12 | 2/5 | 0/18 | 5/10 | 0/7 | 5/5 | 0/5 | |||||||
| Probes in region, Affymetrix 6.0/ Illumina 610K/Illumina 550Kf | 92/32/19 | 94/28/20 | 26/15/15 | 55/14/—e | 292/131/124 | 855/296/261 | 28/5/6 | |||||||
| Groupb,c or Analysis | 1q21.1 Deletions, 144.6-46.3 Mb | 1q21.1 Duplications, 144.6-46.3 Mb | 15q13.3 Deletions, 28.7-30.3 Mb | 22q11.21 Deletions, 17.1-20.2 Mb | 16p11.2 Duplications, 29.5-30.1 Mb | 16p11.2 Deletions, 29.5-30.1 Mb | NRXN1 Exonic Deletions, Chromosome 2, 50. 0-51.1 Mb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| With | Without | With | Without | With | Without | With | Without | With | Without | With | Without | With | Without | |
| MGS total case subjects (current study) | 4 | 3,941 | 7 | 3,938 | 7 | 3,938 | 21 | 3,924 | 13 | 3,932 | 1 | 3,944 | 10 | 3,935 |
| MGS total comparison subjects (current study) | 1 | 3,610 | 0 | 3,611 | 1 | 3,610 | 0 | 3,611 | 1 | 3,610 | 3 | 3,608 | 1 | 3,610 |
| MGS European-ancestry case subjects | 2 | 2,669 | 5 | 2,666 | 7 | 2,664 | 18 | 2,653 | 10 | 2,661 | 1 | 2,670 | 9 | 2,662 |
| MGS European-ancestry comparison subjects | 1 | 2,647 | 0 | 2,648 | 1 | 2,647 | 0 | 2,648 | 0 | 2,648 | 3 | 2,645 | 1 | 2,647 |
| MGS African American case subjects | 2 | 1,272 | 2 | 1,272 | 0 | 1,274 | 3 | 1,271 | 3 | 1,271 | 0 | 1,274 | 1 | 1,273 |
| MGS African American comparison subjects | 0 | 963 | 0 | 963 | 0 | 963 | 0 | 963 | 1 | 962 | 0 | 963 | 0 | 963 |
| ISC case subjects | 9 | 3,382 | 3 | 3,388 | 8 | 3,383 | 11 | 3,380 | 6 | 3,385 | 1 | 3,390 | 3 | 3,388 |
| ISC comparison subjects | 1 | 3,180 | 1 | 3,180 | 0 | 3,181 | 0 | 3,181 | 1 | 3,180 | 3 | 3,178 | 1 | 3,180 |
| deCODE (1) Iceland case subjects | 1 | 645 | 1 | 647 | 1 | 645 | 1 | 645 | — | — | — | — | — | — |
| deCODE (1) Iceland comparison subjects | 8 | 32,434 | 12 | 32,430 | 7 | 32,435 | 0 | 32,442 | — | — | — | — | — | — |
| deCODE (1) Denmark case subjects | 3 | 439 | — | — | — | — | — | — | — | — | — | — | — | — |
| deCODE (1) Denmark comparison subjects | 0 | 1,437 | — | — | — | — | — | — | — | — | — | — | — | — |
| deCODE (1) the Netherlands case subjects | 0 | 806 | — | — | 3 | 803 | 1 | 805 | — | — | — | — | — | — |
| deCODE (1) the Netherlands comparison subjects (1) | 0 | 4,039 | — | — | 1 | 4,038 | 0 | 4,039 | — | — | — | — | — | — |
| deCODE (1) other case subjects | 3 | 2,159 | 0 | 579 | 2 | 2,097 | 1 | 1,723 | — | — | — | — | — | — |
| deCODE (1) other comparison subjects | 0 | 2,611 | 1 | 574 | 0 | 2,649 | 0 | 2,148 | — | — | — | — | — | — |
| Weiss et al. (20) case subjects | — | — | — | — | — | — | — | — | 0 | 648 | 1 | 647 | — | — |
| Weiss et al. (20) comparison subjects | — | — | — | — | — | — | — | — | 5 | 18,829 | 2 | 18,832 | — | — |
| McCarthy et al. (3)d case subjects | — | — | — | — | — | — | — | — | 12 | 1,894 | 1 | 1,905 | — | — |
| McCarthy et al. (3)d comparison subjects | — | — | — | — | — | — | — | — | 1 | 3,970 | 3 | 3,968 | — | — |
| Walsh et al. (8) case subjects | — | — | — | — | — | — | — | — | — | — | — | — | 1 | 232 |
| Walsh et al. (8) comparison subjects | — | — | — | — | — | — | — | — | — | — | — | — | 0 | 268 |
| Need et al. (21) case subjects | — | — | — | — | — | — | — | — | — | — | — | — | 3 | 1,070 |
| Need et al. (21) comparison subjects | — | — | — | — | — | — | — | — | — | — | — | — | 0 | 1,148 |
| Kirov et al. (4) case subjects | — | — | — | — | — | — | — | — | — | — | — | — | 1 | 470 |
| Kirov et al. (4) comparison subjects | — | — | — | — | — | — | — | — | — | — | — | — | 3 | 2,789 |
| Ikeda et al. (22) case subjects | — | — | — | — | — | — | — | — | — | — | — | — | 0 | 560 |
| Ikeda et al. (22) comparison subjects | — | — | — | — | — | — | — | — | — | — | — | — | 0 | 547 |
| Rujescu et al. (5) case subjects | — | — | — | — | — | — | — | — | — | — | — | — | 5 | 2,972 |
| Rujescu et al. (5) comparison subjects | — | — | — | — | — | — | — | — | — | — | — | — | 5 | 33,741 |
| Total case subjects | 20 | 11,372 | 11 | 8,552 | 21 | 10,866 | 35 | 11,365 | 31 | 9,859 | 4 | 9,886 | 23 | 12,627 |
| Total comparison subjects | 10 | 47,311 | 14 | 39,795 | 9 | 45,913 | 0 | 45,361 | 8 | 29,589 | 11 | 29,586 | 10 | 45,284 |
| Proportion of case subjects | 0.0018 | — | 0.0013 | — | 0.0019 | — | 0.0031 | — | 0.0031 | — | 0.0004 | — | 0.0018 | — |
| Proportion of comparison subjects | 0.0002 | — | 0.0004 | — | 0.0002 | — | 0.0000 | — | 0.0003 | — | 0.0004 | — | 0.0002 | — |
| p | Odds Ratio (CI) | p | Odds Ratio (CI) | p | Odds Ratio (CI) | p | Odds Ratio (CI) | p | Odds Ratio (CI) | p | Odds Ratio (CI) | p | Odds Ratio (CI) | |
| Meta-analysis, Cochran-Mantel-Haenszel exact test | 8.5×10−6 | 0.02 | 6.9×10−7 | 7.3×10−13 | 2.6×10−8 | 0.88 | 1.3×10−6 | |||||||
| One-sided odds ratio (CI, lower bound)e | 9.5 (3.5) | 4.5 (1.4) | 12.1 (4.4) | (20.3) | 9.5 (4.1) | 0.6 (0.2) | 7.5 (3.4) | |||||||
| Two-sided odds ratio (CI) | 9.5 (3.0-33.4) | 4.5 (1.2-17.5) | 12.1 (3.8-42.0) | (15.6-∞) | 9.5 (3.6-28.4) | 0.6 (0.1-2.2) | 7.5 (3.0-19.8) | |||||||
| Pooled analysis, Fisher's exact test | 2.2×10−8 | 0.002 | 2.0×10−9 | >1.0×10−16 | 1.5×10−12 | 0.54 | 5.5×10−9 | |||||||
| One-sided odds ratio (CI, lower bound)e | 8.3 (4.2) | 3.7 (1.7) | 9.9 (4.9) | (44.7) | 11.6 (5.8) | 1.09 (0.32) | 8.2 (4.2) | |||||||
| Two-sided odds ratio (CI) | 8.3 (3.7-19.9) | 3.7 (1.5-8.7) | 9.9 (4.3-24.4) | (35.9-∞) | 11.6 (5.6-29.3) | 1.09 (0.3-3.7) | 8.2 (3.8-19.4) | |||||||
| Clinical Feature | No CNV | Strongest New Findings From Current Study | Confirmed Association With Schizophrenia | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3q29 | VIPR2 | 1q21.1 | NRXN1 | 15q13.3 | 16p11.2 | 22q11.21 | ||||||||||
| N | Proportion | N | Proportion | N | Proportion | N | Proportion | N | Proportion | N | Proportion | N | Proportion | N | Proportion | |
| Group size | 3,766 | 5 | 10 | 4 | 10 | 7 | 13 | 21 | ||||||||
| Male | — | 0.68 | — | 0.40 | — | 0.90 | — | 0.75 | — | 0.70 | — | 0.71 | — | 0.54 | — | 0.52 |
| African American | — | 0.32 | — | 0.20 | — | 0.30 | — | 0.50 | — | 0.10 | — | 0.00 | — | 0.23 | — | 0.14 |
| Schizoaffective disorderb | — | 0.10 | — | 0.00 | — | 0.20 | — | 0.25 | — | 0.20 | — | 0.14 | — | 0.23 | — | 0.05 |
| DSM-IV criteria metb | ||||||||||||||||
| Delusions | — | 0.99 | — | 1.00 | — | 1.00 | — | 1.00 | — | 1.00 | — | 1.00 | — | 1.00 | — | 1.00 |
| Bizarre (impossible) delusions | — | 0.63 | — | 0.60 | — | 0.80 | — | 0.75 | — | 0.60 | — | 0.67 | — | 0.46 | — | 0.52 |
| Hallucinations | — | 0.95 | — | 1.00 | — | 0.90 | — | 0.75 | — | 1.00 | — | 1.00 | — | 1.00 | — | 1.00 |
| Commentary or conversing hallucinations | — | 0.50 | — | 0.40 | — | 0.50 | — | 0.00* | — | 0.40 | — | 0.86 | — | 0.46 | — | 0.52 |
| Formal thought disorder | — | 0.67 | — | 0.60 | — | 0.70 | — | 0.75 | — | 0.80 | — | 0.43 | — | 0.85 | — | 0.52 |
| Disorganized behavior | — | 0.67 | — | 1.00 | — | 0.70 | — | 0.25 | — | 0.70 | — | 0.57 | — | 0.46 | — | 0.62 |
| Negative symptoms | — | 0.83 | — | 0.80 | — | 1.00 | — | 1.00 | — | 0.60* | — | 0.86 | — | 0.77 | — | 0.81 |
| Self-reported comorbid medical conditionsc | ||||||||||||||||
| Thyroid disorder | 355 | 0.10 | 2 | 0.40** | 1 | 0.11 | 1 | 0.25 | 1 | 0.10 | 0 | 0.00 | 1 | 0.08 | 5 | 0.25** |
| Learning problem | 760 | 0.22 | 2 | 0.50 | 3 | 0.38 | 1 | 0.25 | 5 | 0.50** | 3 | 0.43 | 6 | 0.46** | 11 | 0.52*** |
| Seizures | 469 | 0.13 | 2 | 0.40 | 0 | 0.00 | 1 | 0.25 | 4 | 0.40** | 3 | 0.43** | 5 | 0.38** | 6 | 0.29** |
| Number with clinical factor scores | 3,406 | 5 | 8 | 3 | 10 | 7 | 13 | 20 | ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Clinical factor scoresd | ||||||||||||||||
| Positive symptoms | 0.00 | 0.67 | 0.21 | — | 0.50 | — | −0.30 | — | 0.04 | — | −0.09 | — | 0.57+ | — | −0.08 | — |
| Negative and disorganized symptoms | 0.01 | 0.47 | 0.06 | — | 0.22 | — | 0.02 | — | 0.00 | — | −0.22 | — | 0.11 | — | −0.03 | — |
| Mood (depressive and manic) symptoms | 0.14 | 0.74 | 0.15 | — | 0.10 | — | −0.30 | — | 0.79** | — | 0.06 | — | 0.10 | — | 0.11 | — |
| Age at onset (years) | 21.26 | 6.93 | 19.60** | 0.89 | 19.50 | 4.45 | 19.75 | 10.66 | 20.40 | 5.39 | 22.00 | 8.83 | 20.62 | 7.33 | 22.10 | 4.40 |
| Mood comorbiditye | ||||||||||||||||
| Total months of illness (lifetime) | 260 | 137 | 353 | — | 293 | — | 192 | — | 285 | — | 309 | — | 287 | 262 | — | |
| Months with mood syndrome (lifetime) | 18 | 48 | 2† | — | 17 | — | 4** | — | 10* | — | 31 | — | 37 | 24 | — | |
| Proportion of illness with mood syndrome | 0.08 | 0.18 | 0.01† | — | 0.12 | — | 0.13 | — | 0.09 | — | 0.10 | — | 0.12 | 0.08 | — | |
Discussion
Chromosome 1q21.1
Chromosome 15q13.3
Chromosome 16p11.2
Exonic Deletions in NRXN1
Chromosome 22q11.21
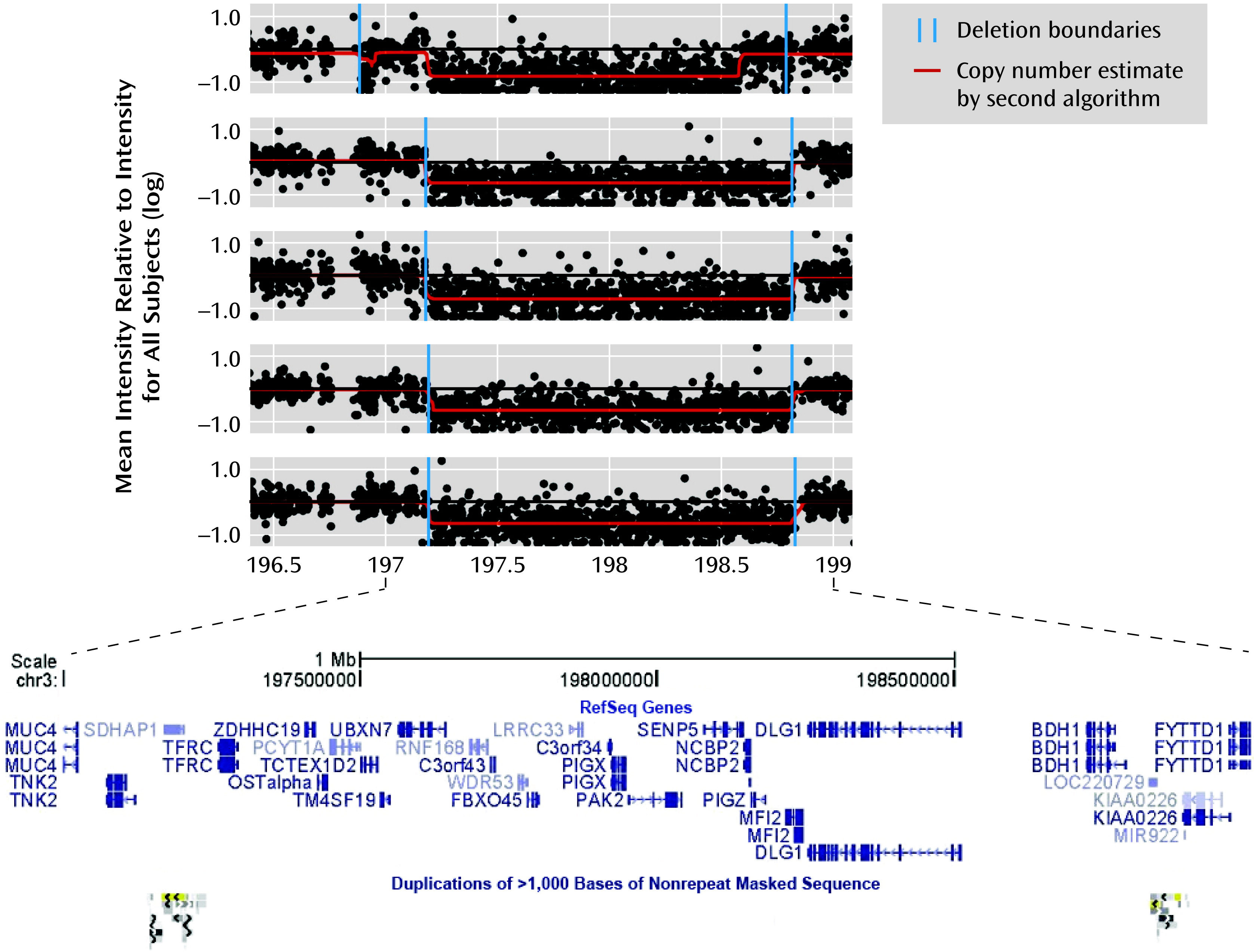
Chromosome 3q29
VIPR2
Other Findings
Genome-Wide CNV Count
Conclusions and Implications for Future Research
Acknowledgments
Footnotes
Supplementary Material
- View/Download
- 1.80 MB
References
Information & Authors
Information
Published In
History
Authors
Funding Information
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

